Research autopsy programmes in oncology: shared experience from 14 centres across the world
Tatjana Geukens, Marion Maetens, Jody E Hooper, Steffi Oesterreich, Adrian V Lee, Lori Miller, Jenny M Atkinson, Margaret Rosenzweig, Shannon Puhalla, Heather Thorne, Lisa Devereux, David Bowtell, Sherene Loi, Eliza R Bacon, Kena Ihle, Mihae Song, Lorna Rodriguez-Rodriguez, Alana L Welm, Lisa Gauchay, Rajmohan Murali, Pharto Chanda, Ali Karacay, Cristina Naceur-Lombardelli, Hayley Bridger, Charles Swanton, Mariam Jamal-Hanjani, Lori Kollath, Lawrence True, Colm Morrissey, Meagan Chambers, Arul M Chinnaiyan, Allecia Wilson, Rohit Mehra, Zachery Reichert, Lisa A Carey, Charles M Perou, Erin Kelly, Daichi Maeda, Akiteru Goto, Janina Kulka, Borbála Székely, A Marcell Szasz, Anna-Mária Tőkés, Wouter Van Den Bogaert, Giuseppe Floris, Christine Desmedt
下载PDF
{"title":"Research autopsy programmes in oncology: shared experience from 14 centres across the world","authors":"Tatjana Geukens, Marion Maetens, Jody E Hooper, Steffi Oesterreich, Adrian V Lee, Lori Miller, Jenny M Atkinson, Margaret Rosenzweig, Shannon Puhalla, Heather Thorne, Lisa Devereux, David Bowtell, Sherene Loi, Eliza R Bacon, Kena Ihle, Mihae Song, Lorna Rodriguez-Rodriguez, Alana L Welm, Lisa Gauchay, Rajmohan Murali, Pharto Chanda, Ali Karacay, Cristina Naceur-Lombardelli, Hayley Bridger, Charles Swanton, Mariam Jamal-Hanjani, Lori Kollath, Lawrence True, Colm Morrissey, Meagan Chambers, Arul M Chinnaiyan, Allecia Wilson, Rohit Mehra, Zachery Reichert, Lisa A Carey, Charles M Perou, Erin Kelly, Daichi Maeda, Akiteru Goto, Janina Kulka, Borbála Székely, A Marcell Szasz, Anna-Mária Tőkés, Wouter Van Den Bogaert, Giuseppe Floris, Christine Desmedt","doi":"10.1002/path.6271","DOIUrl":null,"url":null,"abstract":"<p>While there is a great clinical need to understand the biology of metastatic cancer in order to treat it more effectively, research is hampered by limited sample availability. Research autopsy programmes can crucially advance the field through synchronous, extensive, and high-volume sample collection. However, it remains an underused strategy in translational research. Via an extensive questionnaire, we collected information on the study design, enrolment strategy, study conduct, sample and data management, and challenges and opportunities of research autopsy programmes in oncology worldwide. Fourteen programmes participated in this study. Eight programmes operated 24 h/7 days, resulting in a lower median postmortem interval (time between death and start of the autopsy, 4 h) compared with those operating during working hours (9 h). Most programmes (<i>n</i> = 10) succeeded in collecting all samples within a median of 12 h after death. A large number of tumour sites were sampled during each autopsy (median 15.5 per patient). The median number of samples collected per patient was 58, including different processing methods for tumour samples but also non-tumour tissues and liquid biopsies. Unique biological insights derived from these samples included metastatic progression, treatment resistance, disease heterogeneity, tumour dormancy, interactions with the tumour micro-environment, and tumour representation in liquid biopsies. Tumour patient-derived xenograft (PDX) or organoid (PDO) models were additionally established, allowing for drug discovery and treatment sensitivity assays. Apart from the opportunities and achievements, we also present the challenges related with postmortem sample collections and strategies to overcome them, based on the shared experience of these 14 programmes. Through this work, we hope to increase the transparency of postmortem tissue donation, to encourage and aid the creation of new programmes, and to foster collaborations on these unique sample collections. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 2","pages":"150-165"},"PeriodicalIF":5.6000,"publicationDate":"2024-03-29","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6271","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6271","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
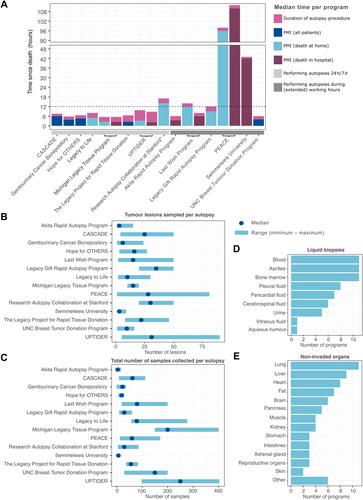
While there is a great clinical need to understand the biology of metastatic cancer in order to treat it more effectively, research is hampered by limited sample availability. Research autopsy programmes can crucially advance the field through synchronous, extensive, and high-volume sample collection. However, it remains an underused strategy in translational research. Via an extensive questionnaire, we collected information on the study design, enrolment strategy, study conduct, sample and data management, and challenges and opportunities of research autopsy programmes in oncology worldwide. Fourteen programmes participated in this study. Eight programmes operated 24 h/7 days, resulting in a lower median postmortem interval (time between death and start of the autopsy, 4 h) compared with those operating during working hours (9 h). Most programmes (n = 10) succeeded in collecting all samples within a median of 12 h after death. A large number of tumour sites were sampled during each autopsy (median 15.5 per patient). The median number of samples collected per patient was 58, including different processing methods for tumour samples but also non-tumour tissues and liquid biopsies. Unique biological insights derived from these samples included metastatic progression, treatment resistance, disease heterogeneity, tumour dormancy, interactions with the tumour micro-environment, and tumour representation in liquid biopsies. Tumour patient-derived xenograft (PDX) or organoid (PDO) models were additionally established, allowing for drug discovery and treatment sensitivity assays. Apart from the opportunities and achievements, we also present the challenges related with postmortem sample collections and strategies to overcome them, based on the shared experience of these 14 programmes. Through this work, we hope to increase the transparency of postmortem tissue donation, to encourage and aid the creation of new programmes, and to foster collaborations on these unique sample collections. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.