Integration of peripheral blood- and tissue-based biomarkers of response to immune checkpoint blockade in urothelial carcinoma
Rami S Vanguri, James W Smithy, Yanyun Li, Mingqiang Zhuang, Colleen A Maher, Nathaniel Aleynick, Xiyu Peng, Hikmat Al-Ahmadie, Samuel A Funt, Jonathan E Rosenberg, Gopa Iyer, Dean Bajorin, James C Mathews, Saad Nadeem, Katherine S Panageas, Ronglai Shen, Margaret K Callahan, Travis J Hollmann
下载PDF
{"title":"Integration of peripheral blood- and tissue-based biomarkers of response to immune checkpoint blockade in urothelial carcinoma","authors":"Rami S Vanguri, James W Smithy, Yanyun Li, Mingqiang Zhuang, Colleen A Maher, Nathaniel Aleynick, Xiyu Peng, Hikmat Al-Ahmadie, Samuel A Funt, Jonathan E Rosenberg, Gopa Iyer, Dean Bajorin, James C Mathews, Saad Nadeem, Katherine S Panageas, Ronglai Shen, Margaret K Callahan, Travis J Hollmann","doi":"10.1002/path.6197","DOIUrl":null,"url":null,"abstract":"<p>As predictive biomarkers of response to immune checkpoint inhibitors (ICIs) remain a major unmet clinical need in patients with urothelial carcinoma (UC), we sought to identify tissue-based immune biomarkers of clinical benefit to ICIs using multiplex immunofluorescence and to integrate these findings with previously identified peripheral blood biomarkers of response. Fifty-five pretreatment and 12 paired on-treatment UC specimens were identified from patients treated with nivolumab with or without ipilimumab. Whole tissue sections were stained with a 12-plex mIF panel, including CD8, PD-1/CD279, PD-L1/CD274, CD68, CD3, CD4, FoxP3, TCF1/7, Ki67, LAG-3, MHC-II/HLA-DR, and pancytokeratin+SOX10 to identify over three million cells. Immune tissue densities were compared to progression-free survival (PFS) and best overall response (BOR) by RECIST version 1.1. Correlation coefficients were calculated between tissue-based and circulating immune populations. The frequency of intratumoral CD3<sup>+</sup>LAG-3<sup>+</sup> cells was higher in responders compared to nonresponders (<i>p</i> = 0.0001). LAG-3<sup>+</sup> cellular aggregates were associated with response, including CD3<sup>+</sup>LAG-3<sup>+</sup> in proximity to CD3<sup>+</sup> (<i>p</i> = 0.01). Exploratory multivariate modeling showed an association between intratumoral CD3<sup>+</sup>LAG-3<sup>+</sup> cells and improved PFS independent of prognostic clinical factors (log HR −7.0; 95% confidence interval [CI] −12.7 to −1.4), as well as established biomarkers predictive of ICI response (log HR −5.0; 95% CI −9.8 to −0.2). Intratumoral LAG-3<sup>+</sup> immune cell populations warrant further study as a predictive biomarker of clinical benefit to ICIs. Differences in LAG-3<sup>+</sup> lymphocyte populations across the intratumoral and peripheral compartments may provide complementary information that could inform the future development of multimodal composite biomarkers of ICI response. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"261 3","pages":"349-360"},"PeriodicalIF":5.2000,"publicationDate":"2023-09-05","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6197","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6197","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
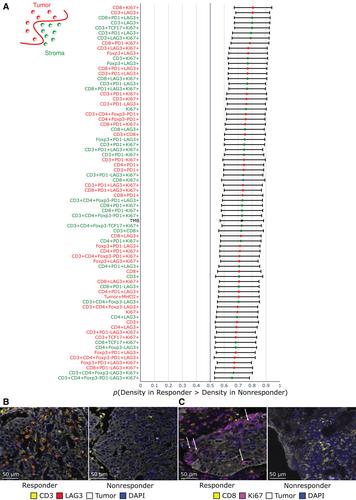
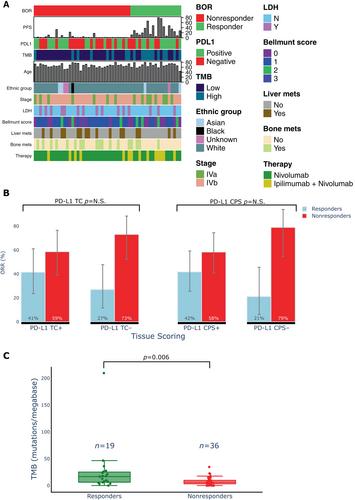
As predictive biomarkers of response to immune checkpoint inhibitors (ICIs) remain a major unmet clinical need in patients with urothelial carcinoma (UC), we sought to identify tissue-based immune biomarkers of clinical benefit to ICIs using multiplex immunofluorescence and to integrate these findings with previously identified peripheral blood biomarkers of response. Fifty-five pretreatment and 12 paired on-treatment UC specimens were identified from patients treated with nivolumab with or without ipilimumab. Whole tissue sections were stained with a 12-plex mIF panel, including CD8, PD-1/CD279, PD-L1/CD274, CD68, CD3, CD4, FoxP3, TCF1/7, Ki67, LAG-3, MHC-II/HLA-DR, and pancytokeratin+SOX10 to identify over three million cells. Immune tissue densities were compared to progression-free survival (PFS) and best overall response (BOR) by RECIST version 1.1. Correlation coefficients were calculated between tissue-based and circulating immune populations. The frequency of intratumoral CD3+ LAG-3+ cells was higher in responders compared to nonresponders (p = 0.0001). LAG-3+ cellular aggregates were associated with response, including CD3+ LAG-3+ in proximity to CD3+ (p = 0.01). Exploratory multivariate modeling showed an association between intratumoral CD3+ LAG-3+ cells and improved PFS independent of prognostic clinical factors (log HR −7.0; 95% confidence interval [CI] −12.7 to −1.4), as well as established biomarkers predictive of ICI response (log HR −5.0; 95% CI −9.8 to −0.2). Intratumoral LAG-3+ immune cell populations warrant further study as a predictive biomarker of clinical benefit to ICIs. Differences in LAG-3+ lymphocyte populations across the intratumoral and peripheral compartments may provide complementary information that could inform the future development of multimodal composite biomarkers of ICI response. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.