Consensus molecular subtype transition during progression of colorectal cancer
Simone van de Weerd, Arezo Torang, Liselotte W Zwager, Pim J Koelink, Jan Koster, Barbara AJ Bastiaansen, Veerle Lammers, Ciro Longobardi, Jeanine ML Roodhart, J Han van Krieken, Arantza Farina Sarasqueta, Evelien Dekker, Jan Paul Medema
下载PDF
{"title":"Consensus molecular subtype transition during progression of colorectal cancer","authors":"Simone van de Weerd, Arezo Torang, Liselotte W Zwager, Pim J Koelink, Jan Koster, Barbara AJ Bastiaansen, Veerle Lammers, Ciro Longobardi, Jeanine ML Roodhart, J Han van Krieken, Arantza Farina Sarasqueta, Evelien Dekker, Jan Paul Medema","doi":"10.1002/path.6176","DOIUrl":null,"url":null,"abstract":"<p>The consensus molecular subtype (CMS) classification divides colorectal cancer (CRC) into four distinct subtypes based on RNA expression profiles. The biological differences between CMSs are already present in CRC precursor lesions, but not all CMSs pose the same risk of malignant transformation. To fully understand the path to malignant transformation and to determine whether CMS is a fixed entity during progression, genomic and transcriptomic data from two regions of the same CRC lesion were compared: the precursor region and the carcinoma region. In total, 24 patients who underwent endoscopic removal of T1–2 CRC were included. Regions were subtyped for CMS and DNA mutation analysis was performed. Additionally, a set of 85 benign adenomas was CMS-subtyped. This analysis revealed that almost all benign adenomas were classified as CMS3 (91.8%). In contrast, CMS2 was the most prevalent subtype in precursor regions (66.7%), followed by CMS3 (29.2%). CMS4 was absent in precursor lesions and originated at the carcinoma stage. Importantly, CMS switching occurred in a substantial number of cases and almost all (six out of seven) CMS3 precursor regions showed a shift to a different subtype in the carcinoma part of the lesion, which in four cases was classified as CMS4. In conclusion, our data indicate that CMS3 is related to a more indolent type of precursor lesion that less likely progresses to CRC and when this occurs, it is often associated with a subtype change that includes the more aggressive mesenchymal CMS4. In contrast, an acquired CMS2 signature appeared to be rather fixed during early CRC development. Combined, our data show that subtype changes occur during progression and that CMS3 switching is related to changes in the genomic background through acquisition of a novel driver mutation (<i>TP53</i>) or selective expansion of a clone, but also occurred independently of such genetic changes. © 2023 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"261 3","pages":"298-308"},"PeriodicalIF":5.6000,"publicationDate":"2023-09-08","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6176","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6176","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
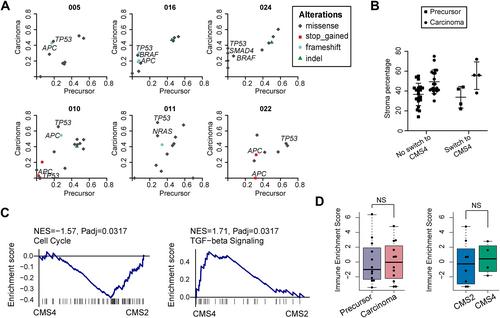
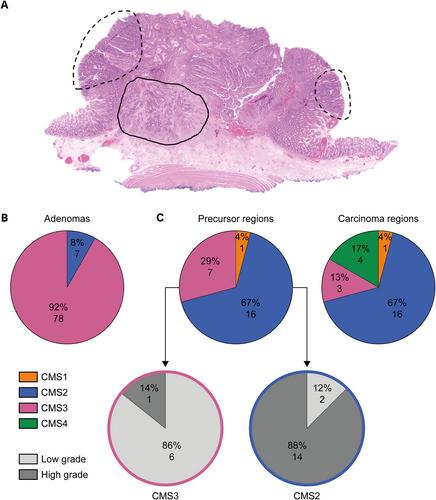
The consensus molecular subtype (CMS) classification divides colorectal cancer (CRC) into four distinct subtypes based on RNA expression profiles. The biological differences between CMSs are already present in CRC precursor lesions, but not all CMSs pose the same risk of malignant transformation. To fully understand the path to malignant transformation and to determine whether CMS is a fixed entity during progression, genomic and transcriptomic data from two regions of the same CRC lesion were compared: the precursor region and the carcinoma region. In total, 24 patients who underwent endoscopic removal of T1–2 CRC were included. Regions were subtyped for CMS and DNA mutation analysis was performed. Additionally, a set of 85 benign adenomas was CMS-subtyped. This analysis revealed that almost all benign adenomas were classified as CMS3 (91.8%). In contrast, CMS2 was the most prevalent subtype in precursor regions (66.7%), followed by CMS3 (29.2%). CMS4 was absent in precursor lesions and originated at the carcinoma stage. Importantly, CMS switching occurred in a substantial number of cases and almost all (six out of seven) CMS3 precursor regions showed a shift to a different subtype in the carcinoma part of the lesion, which in four cases was classified as CMS4. In conclusion, our data indicate that CMS3 is related to a more indolent type of precursor lesion that less likely progresses to CRC and when this occurs, it is often associated with a subtype change that includes the more aggressive mesenchymal CMS4. In contrast, an acquired CMS2 signature appeared to be rather fixed during early CRC development. Combined, our data show that subtype changes occur during progression and that CMS3 switching is related to changes in the genomic background through acquisition of a novel driver mutation (TP53 ) or selective expansion of a clone, but also occurred independently of such genetic changes. © 2023 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.