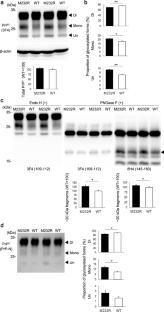
A missense variant from methionine to arginine at codon 232 (M232R) of the prion protein gene accounts for ~ 15% of Japanese patients with genetic prion diseases. However, pathogenic roles of the M232R substitution for the induction of prion disease have remained elusive because family history is usually absent in patients with M232R. In addition, the clinicopathologic phenotypes of patients with M232R are indistinguishable from those of sporadic Creutzfeldt-Jakob disease patients. Furthermore, the M232R substitution is located in the glycosylphosphatidylinositol (GPI)-attachment signal peptide that is cleaved off during the maturation of prion proteins. Therefore, there has been an argument that the M232R substitution might be an uncommon polymorphism rather than a pathogenic mutation. To unveil the role of the M232R substitution in the GPI-attachment signal peptide of prion protein in the pathogenesis of prion disease, here we generated a mouse model expressing human prion proteins with M232R and investigated the susceptibility to prion disease. The M232R substitution accelerates the development of prion disease in a prion strain-dependent manner, without affecting prion strain-specific histopathologic and biochemical features. The M232R substitution did not alter the attachment of GPI nor GPI-attachment site. Instead, the substitution altered endoplasmic reticulum translocation pathway of prion proteins by reducing the hydrophobicity of the GPI-attachment signal peptide, resulting in the reduction of N-linked glycosylation and GPI glycosylation of prion proteins. To the best of our knowledge, this is the first time to show a direct relationship between a point mutation in the GPI-attachment signal peptide and the development of disease.