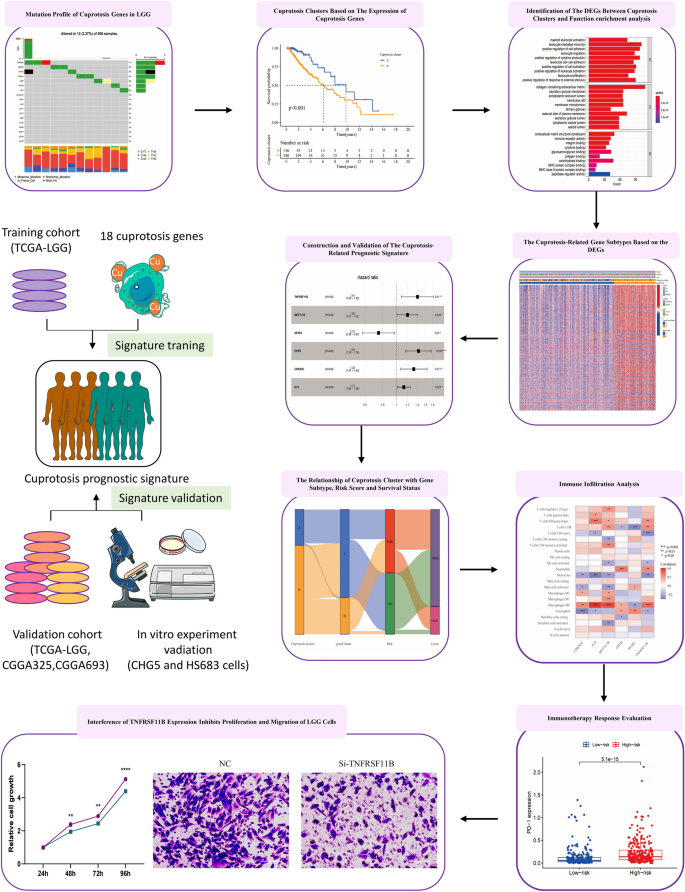
Cuprotosis, an emerging mode of cell death, has recently caught the attention of researchers worldwide. However, its impact on low-grade glioma (LGG) patients has not been fully explored. To gain a deeper insight into the relationship between cuprotosis and LGG patients’ prognosis, we conducted this study in which LGG patients were divided into two clusters based on the expression of 18 cuprotosis-related genes. We found that LGG patients in cluster A had better prognosis than those in cluster B. The two clusters also differed in terms of immune cell infiltration and biological functions. Moreover, we identified differentially expressed genes (DEGs) between the two clusters and developed a cuprotosis-related prognostic signature through the least absolute shrinkage and selection operator (LASSO) analysis in the TCGA training cohort. This signature divided LGG patients into high- and low-risk groups, with the high-risk group having significantly shorter overall survival (OS) time than the low-risk group. Its predictive reliability for prognosis in LGG patients was confirmed by the TCGA internal validation cohort, CGGA325 cohort and CGGA693 cohort. Additionally, a nomogram was used to predict the 1-, 3-, and 5-year OS rates of each patient. The analysis of immune checkpoints and tumor mutation burden (TMB) has revealed that individuals belonging to high-risk groups have a greater chance of benefiting from immunotherapy. Functional experiments confirmed that interfering with the signature gene TNFRSF11B inhibited LGG cell proliferation and migration. Overall, this study shed light on the importance of cuprotosis in LGG patient prognosis. The cuprotosis-related prognostic signature is a reliable predictor for patient outcomes and immunotherapeutic response and can help to develop new therapies for LGG.