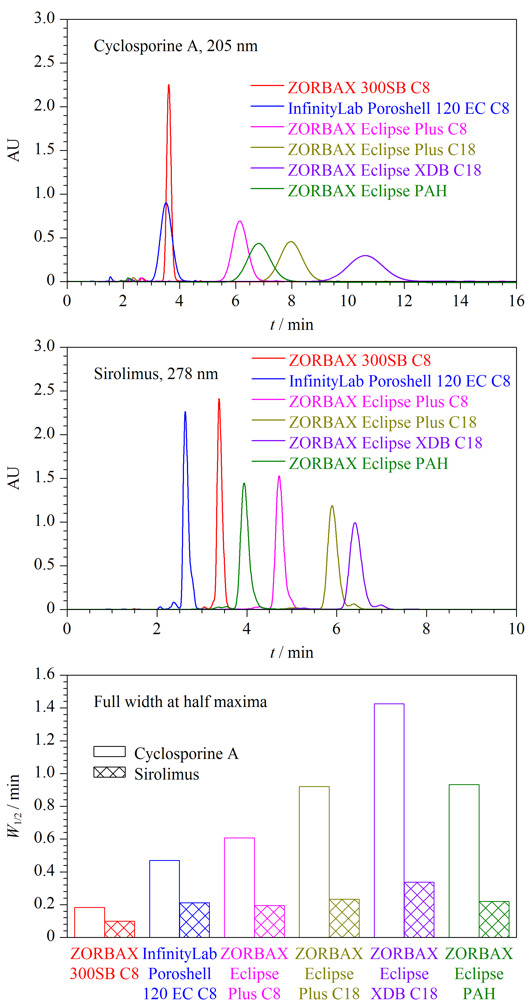
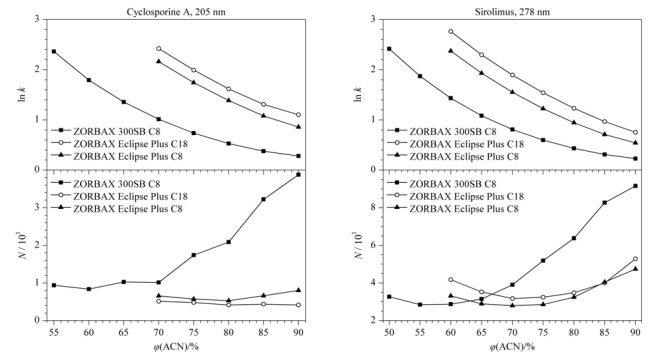
Cyclosporine A and sirolimus are immunosuppressants that are widely used in many organ transplantation procedures. They exhibit some complementary mechanisms of action and interact synergistically when used together. However, they are critical-dose drugs and have a narrow therapeutic index. They provide the desired therapeutic effect with acceptable tolerability only within a specific range of blood concentrations. Therefore, the rapid and simultaneous detection of the concentrations of cyclosporine A and sirolimus in whole blood could provide valuable information on planning medicine administration after organ transplantations. In this study, firstly, the chromatographic behaviors of cyclosporine A and sirolimus on a biological liquid chromatography (BioLC) column and traditional liquid chromatography (TraLC) columns were investigated systematically under the same chromatographic conditions. The results suggested that the peak height and peak width of cyclosporine A and sirolimus on the BioLC column, ZORBAX 300SB C8 (250 mm×4.6 mm, 5.0 μm), were the highest and narrowest, respectively. The number of theoretical plates of cyclosporine A and sirolimus on the ZORBAX 300SB C8 column increased significantly when the volume ratio of acetonitrile in the mobile phases was greater than 70%. Their retention time on the BioLC and TraLC columns was negligibly affected by the use of formic acid and trifluoroacetic acid as the mobile phases. In the range of the experimental column temperature, the number of theoretical plates of cyclosporine A and sirolimus on the ZORBAX 300SB C8 column was significantly higher than that on the two TraLC columns. Furthermore, the relationship between the retention factor and column temperature of cyclosporine A on the ZORBAX 300SB C8 column was different from that on the two TraLC columns. Subsequently, a high performance liquid chromatography method based on the ZORBAX 300SB C8 column was established for the rapid separation and determination of cyclosporin A and sirolimus in whole blood. A sample of whole blood with a volume of 50 μL was prepared by protein precipitation with 1 mol/L sodium hydroxide and then extracted into 500 μL of ether-methanol (95∶5, v/v). After centrifugation at 14000 r/min for 10 min, the organic layer was removed and evaporated under a stream of nitrogen at 50 ℃. The residue was then reconstituted in 200 μL of methanol for use. Cyclosporin A and sirolimus were separated through isocratic elution on the ZORBAX 300SB C8 column. The column temperature was set at 60 ℃. The mobile phase was acetonitrile-water (70∶30, v/v) and the flow rate was 1.0 mL/min. The detection wavelengths were 205 nm for cyclosporine A and 278 nm for sirolimus. The injection volume was 20 μL. The external standard method was used to quantify cyclosporine A and sirolimus. Under the optimized conditions, cyclosporine A and sirolimus were well-separated within 6 min with a resolution of 3.7 at 205 nm. In addition, the endogenous substances in whole blood negligibly interfered in the detection of sirolimus, while two endogenous substances slightly affected the detection of cyclosporine A. Cyclosporine A and sirolimus both showed good linear relationships in their respective concentration (r>0.997). The limits of detection (LODs) of cyclosporine A and sirolimus were respectively calculated to be 10 ng/mL and 1 ng/mL based on a signal-to-noise ratio of 3, and the limits of quantification (LOQs) were 30 ng/mL and 2 ng/mL based on a signal-to-noise ratio of 10. In the whole blood samples, the recoveries of cyclosporine A and sirolimus at three spiked levels were in the ranges of 83.5%-89.7% and 95.8%-97.8% with relative standard deviations (RSDs) of 3.2%-9.0% and 3.4%-6.7% (n=5), respectively. The established method is simple in operation, can be performed with a simple mobile phase, has a short analysis time, and provides a wide linear range and high sensitivity; hence, it can be applied to the determination of cyclosporine A and sirolimus in whole blood.