Over the past decade, successive outbreaks and epidemics of infectious diseases have challenged the emergency preparedness and response systems of global public health institutions, a context in which vaccines have become the centerpiece to strengthening global health security. Nevertheless, vaccine research and development (R&D) is a complex, lengthy, risky, uncertain, and expensive process. Alongside strict, time-consuming regulatory compliance, it takes multiple candidates and many years to register a new vaccine. This is certainly not welcome in a global health crisis such as the COVID-19 pandemic. Therefore, this study aims to understand the R&D paradigm shift in pandemic contexts and its impacts on the value chain of vaccine innovation.
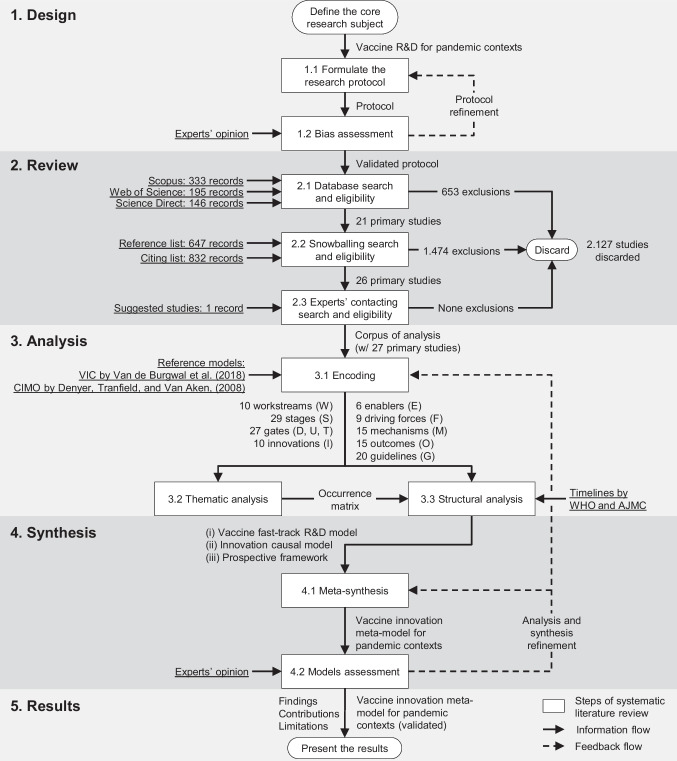
To that end, this paper carried out a systematic literature review and meta-synthesis of 27 articles and reports (2011–2021) that addressed vaccine R&D in contexts of global health threats, disease outbreaks, epidemics, or pandemics.
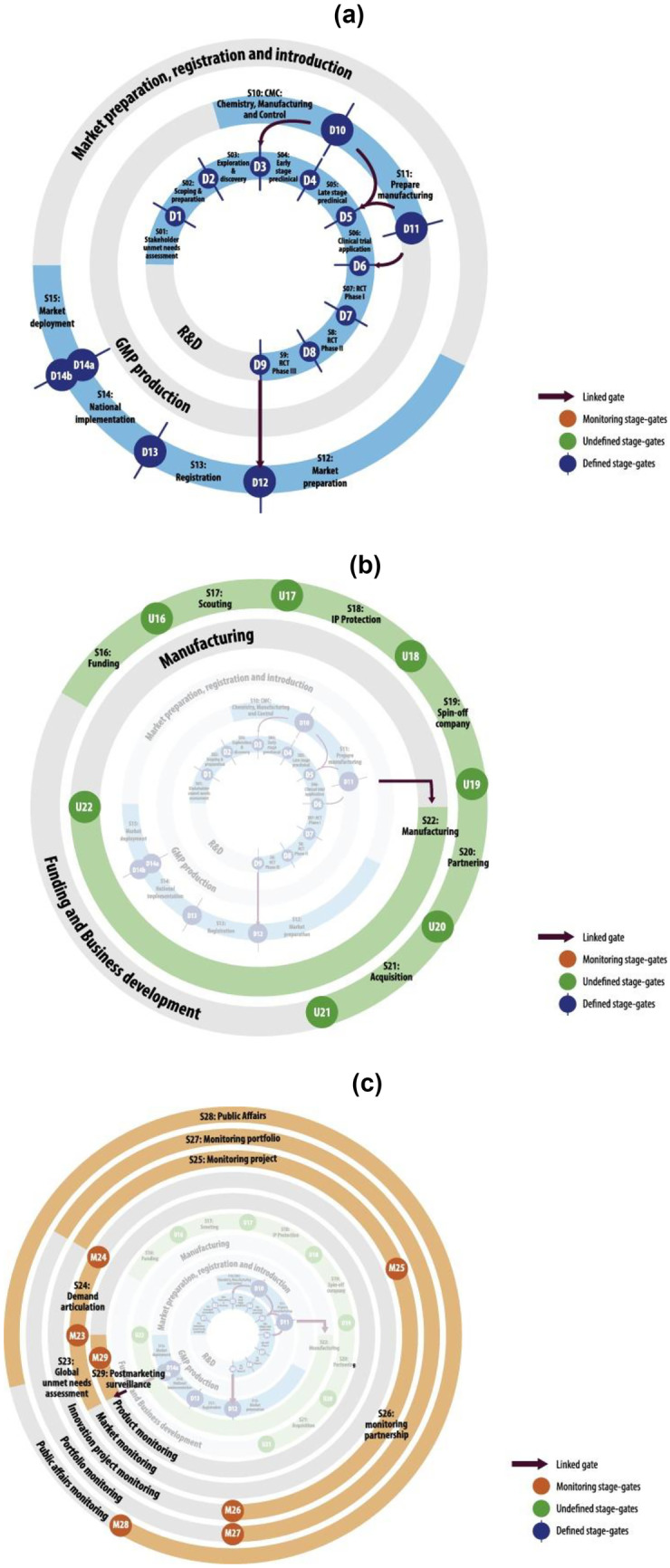
The research findings are synthesized in a meta-model, which describes a fast-track R&D for pandemic contexts, its driving forces, innovations, mechanisms, and impacts in the value chain of vaccine innovation.
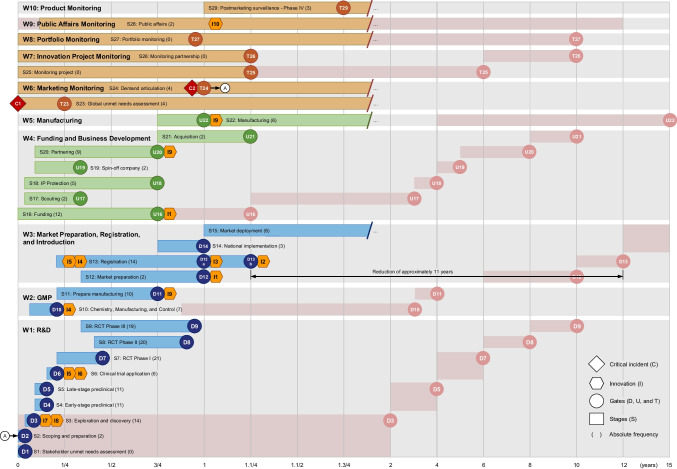
The study demonstrates that, in pandemic contexts, a fast-track R&D process based on close collaboration among regulators, industry, and academia and leveraging enabling technologies can drastically reduce the time required to bring safe, stable, and effective vaccines to market by an average of 11 years compared to the traditional R&D process. Furthermore, pharmacovigilance and rigorous monitoring of real-world evidence became critical to ensuring that quality and safe products were authorized for use during a pandemic.