Immune cell homeostasis plays a crucial role in cancer research and therapeutic response. While chemotherapy and immunotherapy hold promise in treating osteosarcoma (OS), identifying patients who are likely to respond would significantly improve clinical practices. Necroptosis, a fundamental mechanism mediating chemotherapy and immunotherapy efficacy, offers valuable insights. In this context, subtypes based on necroptosis-related genes have been established to predict the response of OS patients to immunotherapy and chemotherapy.
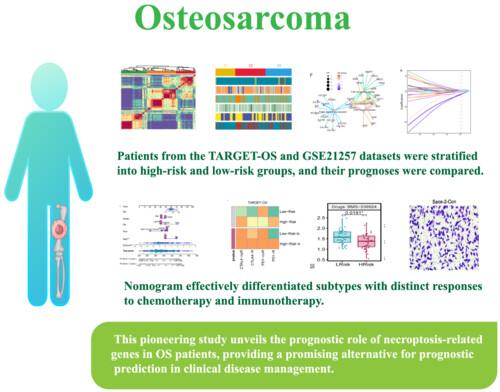
We conducted a high-throughput screening test to identify necroptosis-associated genes that regulate the development of osteosarcoma. Subsequently, the ConsensusClusterPlus package was employed to classify OS patients into subtypes, enabling comparisons of prognosis and clinical information between these subtypes. Patients from the TARGET-OS and GSE21257 datasets were stratified into high-risk and low-risk groups, and their prognoses were compared. Additionally, we assessed the accuracy of the Risk Scoring Model in predicting prognosis, identified independent prognostic factors and explored potential chemotherapeutic agents and immunotherapy drugs.
Through the intersection of expression profiles from the TARGET-OS and GSE21257 datasets, we have identified a total of 92 genes associated with necroptosis. Based on differences in the expression of these genes, patients were divided into three subtypes, and we investigated the differences in tumor-infiltrating immune cells, immune-related pathways, and prognosis among these subtypes. Our nomogram effectively differentiated subtypes with distinct responses to chemotherapy and immunotherapy. The established signature demonstrated superior prediction ability compared with single clinical indicators.
This pioneering study unveils the prognostic role of necroptosis-related genes in OS patients, providing a promising alternative for prognostic prediction in clinical disease management. Moreover, our findings highlight the significance of immune cell homeostasis in cancer research and therapeutic response, underscoring its relevance in advancing current treatment strategies.