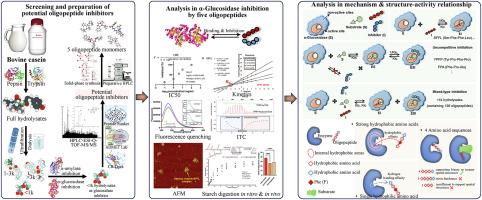
The casein hydrolysate peptides that had inhibitory activity against starch-hydrolyzing enzymes were explored and screened. After enzymolysis, dialysis, ultrafiltration, and lyophilization, hydrolysate peptides with molecular weight >3 k, 1-3 k and <1 k Da were obtained. The hydrolysates were determined with inhibitory activity against α-glucosidase, instead of α-amylase, and the active components concentrated in <1 k hydrolysates. By combination of HPLC-ESI-Q-TOF-MS/MS analysis, systematical evaluation, and simulated docking, 5 oligopeptides were screened from <1 k hydrolysates as the potential inhibitors of α-glucosidase, including SFFL, YPFP, PFA, LYGF and GPFPI. Then, 5 oligopeptide monomers were obtained through solid-phase synthesis and HPLC purification for inhibitory activity confirmation and mechanism elucidation. It was found that the inhibitory activity of the screened oligopeptides were significantly stronger than <1 k hydrolysates, with the intensity order of SFFL > LYGF ≈ YPFP ≈ GPFPI > FPA. The competitive inhibition character of SFFL and the uncompetitive inhibition characters of YPFP and FPA contributed to the mixed-type inhibition model of <1 k hydrolysates. SFFL could bind with the active site of α-glucosidase, forming the specific oligopeptide-enzyme binary complex. YPFP and FPA tended to bind with the enzyme-substrate, forming the oligopeptide-enzyme-substrate ternary complex, instead of directly binding with the enzyme. Considering the structure-activity relationship, the intensive hydrophobic amino acids, single hydrophilic amino acid, and four amino acid sequences favored the oligopeptides to interact with α-glucosidase through hydrophobic interactions, hydrogen bondings and ionic interactions. Due to the enzyme inhibition, the hydrolysate peptides could retard starch digestion both in vitro and in vivo, making it as a potential functional component for regulation of postprandial blood glucose level.
| 公司名称 | 产品信息 | 采购帮参考价格 | |
|---|---|---|---|
| 阿拉丁 | trypsin | ¥28.00~¥311508.69 | |
| 阿拉丁 | DMSO | ¥20.00~¥221931.13 | |
| 阿拉丁 | pepsin | ¥32.00~¥98370.53 | |
| 阿拉丁 | anhydrous sodium carbonate | ¥16.00~¥44404.31 | |
| 阿拉丁 | sodium hydroxide | ¥15.00~¥24697.17 | |
| 上海源叶 | α-Glucosidase from Saccharomyces cerevisiae | ¥348.00~¥10600.00 | |
| 阿拉丁 | hydrochloric acid | ¥16.00~¥5635.43 | |
| 上海源叶 | p -nitrophenyl-α- d -glucopyranoside (pNPG) | ||
| 上海源叶 | α -Glucosidase from Saccharomyces cerevisiae | ||
| 上海源叶 | p -nitrophenyl-α -d -glucopyranoside |
|
|
| 阿拉丁 | Bovine milk casein |
|
|
| 阿拉丁 | Bovine milk casein |
|


