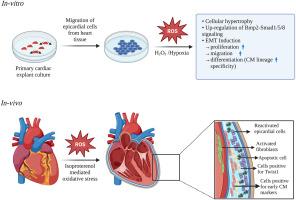
Heart failure has become a major life-threatening cause affecting millions globally, characterized by the permanent loss of adult functional cardiomyocytes leading to fibrosis which ultimately deprives the heart of its functional efficacy. Here we investigated the reparative property of embryonic and adult epicardial cells towards cardiomyocyte differentiation under oxidative stress-induced conditions along with the identification of a possible molecular signaling pathway. Isolated epicardial cells from embryonic chick hearts subjected to oxidative stress and hypoxia induction. Initial assessment of successful injury induction reveals hypertrophy of isolated epicardial cells. Detailed marker gene expression analyses and inhibitor studies reveal Bone morphogenic protein (Bmp)2-Smad1/5/8 signaling dependent cardiomyocyte lineage specification via epithelial to mesenchymal transition (EMT) post-injury. EMT is further confirmed by increased proliferation, migration, and differentiation towards cardiomyocyte lineage. We have also established an in-vivo model in adult male rats using Isoproterenol. Successful oxidative stress-mediated injury induction in adult heart was marked by increased activated fibroblasts followed by apoptosis of adult cardiomyocytes. The detailed characterization of adult epicardial cells reveals similar findings to our avian in-vitro data. Both in-vitro and in-vivo results show a significant increase in the expression of cardiomyocyte specific markers indicative of lineage specificity and activation of epicardial cells post oxidative stress mediated injury. Our findings suggest an EMT-induced reactivation of epicardial cells and early cardiomyocyte lineage specification following oxidative stress in a Bmp2- Smad1/5/8 dependent manner. Overall, this regulatory mechanism of cardiomyocyte differentiation induced by oxidative stress may contribute to the field of cardiac repair and regenerative therapeutics.