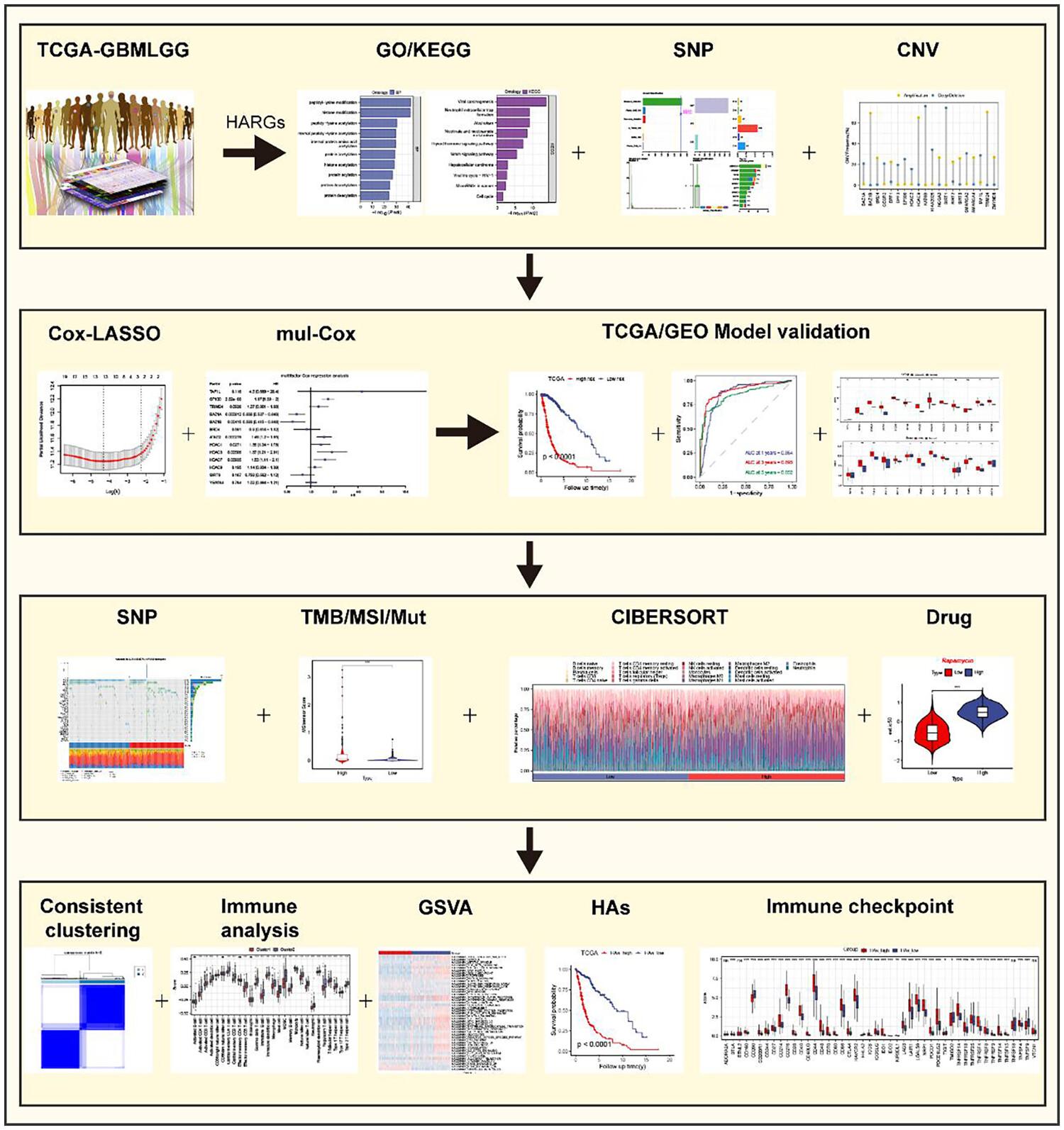
The purpose of this research was to study the impact of histone acetylation on glioblastoma multiforme (GBM) and lower-grade gliomas (LGG) and its potential implications for patient prognosis. We aimed to assess the histone acetylation score (HAs) and its relationship with key genes involved in histone acetylation regulation.
The TCGA-GBMLGG dataset, which provides comprehensive genomic and clinical information, was utilized for this study. We calculated the HAs by analyzing the expression levels of histone acetylation-related genes, including histone acetyltransferases and histone deacetylases, in GBM and LGG patients. Kaplan–Meier survival analysis was performed to evaluate the prognostic value of the HAs. Furthermore, correlation analysis and differential expression analysis were conducted to assess the relationship between the HAs and key genes involved in histone acetylation regulation, as well as the expression differences of immune checkpoint genes.
Our analysis revealed a significant association between the HAs and patient prognosis, with higher HAs correlating to poorer outcomes in GBM and LGG patients. We observed a positive correlation between the HAs and key genes involved in histone acetylation regulation, indicating their potential role in modulating histone acetylation levels. Moreover, we found significant expression differences for immune checkpoint genes between high and low HAs groups, suggesting a potential impact of histone acetylation on the immune response in GBM and LGG.
This study highlights the significance of histone acetylation in GBM and LGG. The HAs demonstrated prognostic value, indicating its potential as a clinically relevant biomarker. The correlation between the HAs and key genes involved in histone acetylation regulation provides insights into the underlying mechanisms driving histone acetylation dysregulation in GBM and LGG. Furthermore, the observed expression differences of immune checkpoint genes suggest a potential link between histone acetylation and the immune response. These findings contribute to our understanding of the molecular basis of GBM and LGG and have implications for personalized treatment approaches targeting histone acetylation and the immune microenvironment. Further validation and functional studies are needed to confirm these findings and explore potential therapeutic strategies.