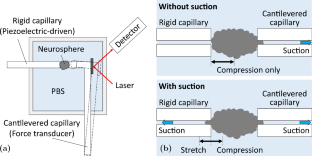
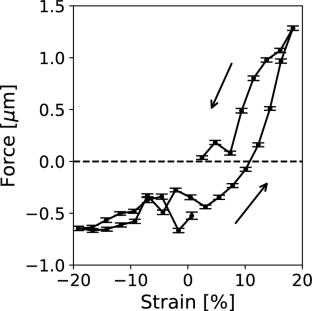
The formulation of more accurate models to describe tissue mechanics necessitates the availability of tools and instruments that can precisely measure the mechanical response of tissues to physical loads and other stimuli. In this regard, neuroscience has trailed other life sciences owing to the unavailability of representative live tissue models and deficiency of experimentation tools. We previously addressed both challenges by employing a novel instrument called the cantilevered-capillary force apparatus (CCFA) to elucidate the mechanical properties of mouse neurospheres under compressive forces. The neurospheres were derived from murine stem cells, and our study was the first of its kind to investigate the viscoelasticity of living neural tissues in vitro. In the current study, we demonstrate the utility of the CCFA as a broadly applicable tool to evaluate tissue mechanics by quantifying the effect that oxidative stress has on the mechanical properties of neurospheres. We treated mouse neurospheres with non-cytotoxic levels of hydrogen peroxide and subsequently evaluated the storage and loss moduli of the tissues under compression and tension. We observed that the neurospheres exhibit viscoelasticity consistent with neural tissue and show that elastic modulus decreases with increasing size of the neurosphere. Our study yields insights for establishing rheological measurements as biomarkers by laying the groundwork for measurement techniques and showing that the influence of a particular treatment may be misinterpreted if the size dependence is ignored.