Co-targeting BET, CBP, and p300 inhibits neuroendocrine signalling in androgen receptor-null prostate cancer
Nicholas Choo, Shivakumar Keerthikumar, Susanne Ramm, Daisaku Ashikari, Linda Teng, Birunthi Niranjan, Shelley Hedwards, Laura H Porter, David L Goode, Kaylene J Simpson, Renea A Taylor, Gail P Risbridger, Mitchell G Lawrence
下载PDF
{"title":"Co-targeting BET, CBP, and p300 inhibits neuroendocrine signalling in androgen receptor-null prostate cancer","authors":"Nicholas Choo, Shivakumar Keerthikumar, Susanne Ramm, Daisaku Ashikari, Linda Teng, Birunthi Niranjan, Shelley Hedwards, Laura H Porter, David L Goode, Kaylene J Simpson, Renea A Taylor, Gail P Risbridger, Mitchell G Lawrence","doi":"10.1002/path.6280","DOIUrl":null,"url":null,"abstract":"<p>There are diverse phenotypes of castration-resistant prostate cancer, including neuroendocrine disease, that vary in their sensitivity to drug treatment. The efficacy of BET and CBP/p300 inhibitors in prostate cancer is attributed, at least in part, to their ability to decrease androgen receptor (AR) signalling. However, the activity of BET and CBP/p300 inhibitors in prostate cancers that lack the AR is unclear. In this study, we showed that BRD4, CBP, and p300 were co-expressed in AR-positive and AR-null prostate cancer. A combined inhibitor of these three proteins, NEO2734, reduced the growth of both AR-positive and AR-null organoids, as measured by changes in viability, size, and composition. NEO2734 treatment caused consistent transcriptional downregulation of cell cycle pathways. In neuroendocrine models, NEO2734 treatment reduced <i>ASCL1</i> levels and other neuroendocrine markers, and reduced tumour growth <i>in vivo</i>. Collectively, these results show that epigenome-targeted inhibitors cause decreased growth and phenotype-dependent disruption of lineage regulators in neuroendocrine prostate cancer, warranting further development of compounds with this activity in the clinic. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 2","pages":"242-256"},"PeriodicalIF":5.6000,"publicationDate":"2024-04-05","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6280","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6280","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
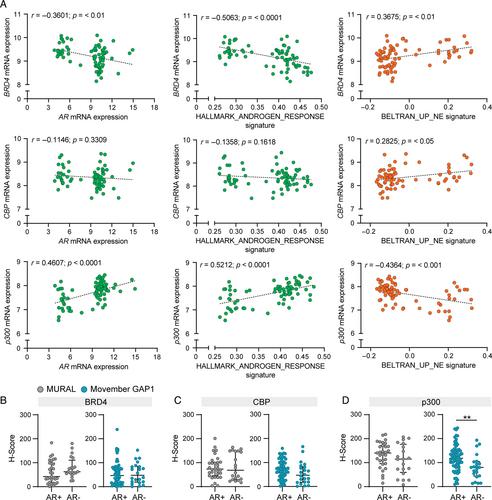
There are diverse phenotypes of castration-resistant prostate cancer, including neuroendocrine disease, that vary in their sensitivity to drug treatment. The efficacy of BET and CBP/p300 inhibitors in prostate cancer is attributed, at least in part, to their ability to decrease androgen receptor (AR) signalling. However, the activity of BET and CBP/p300 inhibitors in prostate cancers that lack the AR is unclear. In this study, we showed that BRD4, CBP, and p300 were co-expressed in AR-positive and AR-null prostate cancer. A combined inhibitor of these three proteins, NEO2734, reduced the growth of both AR-positive and AR-null organoids, as measured by changes in viability, size, and composition. NEO2734 treatment caused consistent transcriptional downregulation of cell cycle pathways. In neuroendocrine models, NEO2734 treatment reduced ASCL1 levels and other neuroendocrine markers, and reduced tumour growth in vivo . Collectively, these results show that epigenome-targeted inhibitors cause decreased growth and phenotype-dependent disruption of lineage regulators in neuroendocrine prostate cancer, warranting further development of compounds with this activity in the clinic. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.