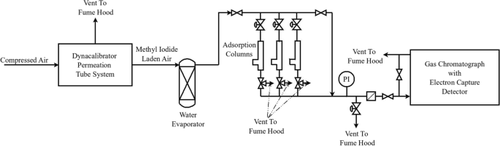
Reduced silver mordenite has been considered as a sorbent for the capture of organic iodides, especially methyl iodide, from off-gases produced by aqueous used nuclear fuel reprocessing operations. The adsorption capacity of this material has been unpredictable especially when NOx and water are present. Previous work has found that a catalytic decomposition reaction is occurring on the surface but few determinations have been made of the kinetics of this reaction. The work presented tested the adsorption behavior and apparent catalytic reaction rate in humid conditions and compared those to dry conditions testing. Both experiments observed a first order reaction with rate constants of 0.0847 L/g sorbent/s and 0.1202 L/g sorbent/s respectively. Such a reduction in apparent rate constant is possibly due to either water obstructing methyl iodide adsorption or product desorption limitation. Changes in the adsorption profile were also apparent between these two, with the humid conditions experiment reaching saturation sooner than the dry conditions experiment. Additionally, an experiment into the effects of sorbent storage in a controlled laboratory environment was performed. The performance of the sorbent materials that were stored with silver in the zerovalent state was slightly inferior to those materials that were stored in ionic form (Ag+) and reduced to zerovalent silver immediately prior to subjecting them to sorption test. The materials stored with silver in the ionic form (and reduced just prior to application) behaved essentially similarly to the freshly synthesized (and reduced) sorbents in the sorption tests. This suggests that zerovalent silver experiences some oxidation resulting in deactivation of some sites.