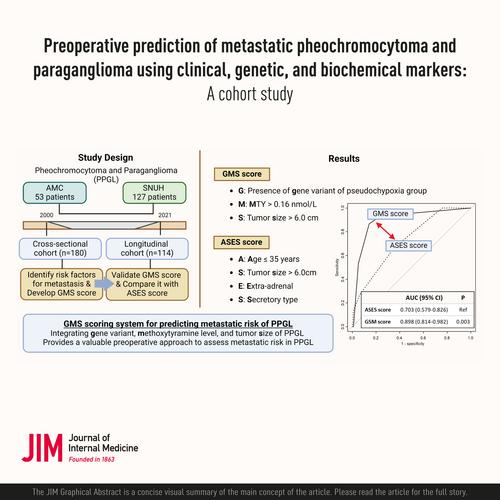
The prevalence of metastatic pheochromocytoma and paraganglioma (PPGL) is approximately 15%–20%. Although there are indicators to assess metastatic risks, none of them predict metastasis reliably. Therefore, we aimed to develop and validate a scoring system using clinical, genetic, and biochemical risk factors to preoperatively predict the metastatic risk of PPGL.
In the cross-sectional cohort (n = 180), clinical, genetic, and biochemical risk factors for metastasis were identified using multivariate logistic regression analysis, and a novel scoring system was developed. The scoring system was validated and compared with the age, size of tumor, extra-adrenal location, and secretory type (ASES) score in the longitudinal cohort (n = 114).
In the cross-sectional cohort, pseudohypoxia group-related gene variants (SDHB, SDHD, or VHL), methoxytyramine >0.16 nmol/L, and tumor size >6.0 cm were independently associated with metastasis after multivariate logistic regression. Using them, the gene variant, methoxytyramine, and size of tumor (GMS) score were developed. In the longitudinal cohort, Harrell's concordance index of the GMS score (0.873, 95% confidence interval [CI]: 0.738–0.941) was higher than that of the ASES score (0.713, 95% CI: 0.567–0.814, p = 0.007). In the longitudinal cohort, a GMS score ≥2 was significantly associated with a higher risk of metastasis (hazard ratio = 25.07, 95% CI: 5.65–111.20). A GMS score ≥2 (p < 0.001), but not ASES score ≥2 (p = 0.090), was associated with shorter progression-free survival.
The GMS scoring system, which integrates gene variant, methoxytyramine level, and tumor size, provides a valuable preoperative approach to assess metastatic risk in PPGL.