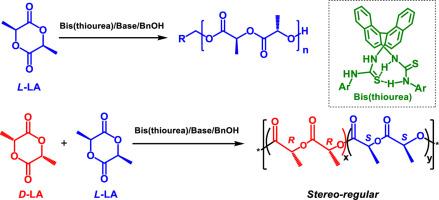
Development of H-bonding organo-catalysts with high catalytic activity and high degree of control is significantly important for the metal-free polyesters that are potentially suitable for biomedical usage. The concomitant use of base and commercially available thiourea generally suffered from prolonged reaction time and resulted in incomplete monomer conversion in some cases. Introducing one or more (thio)urea H-bonding donating arms to the parent thiourea has been proved to be an effective method for substantially increasing the activity of thiourea H-bonding donors. Consequently, we synthesized bis-thioureas derived from binaphthyl-amine bearing different substituents and investigated their catalytic performance in ROPs of lactide in this work. The density functional theory (DFT) calculations suggested that the “activated-thiourea” mode is more preferred for the bis(thiourea) containing binaphthyl-amine framework. The bis(thiourea)/base binary systems could effectively promote the LA polymerization using benzyl alcohol as initiator. The polymerization rates and the degree of control over the polymerization are highly dependent on the structures of the bis(thiourea) and base. Bis(thiourea)/1,5,7-triazabicyclo[4.4.0]dec‑5-ene pairs exhibited highest catalytic activity compared to other bis(thiourea)/base pairs, and the turnover frequency is high up to 1980 h–1 at 75 °C. In addition, the bulky hindrance and axial chirality of the binaphthyl-amine framework could enable stereoselective ROP of rac-LA to produce isotactic-rich and crystalline PLAs at relatively low temperature (0 °C). Mechanistic studies indicated that both enantiomorphic site control (ESC) and chain end control (CEC) concurrently occurred in the rac-LA polymerization catalyzed by bis(thiourea)/base binary systems. This work will inspire future design of H-bonding donors with high catalytic performance.