Camrelizumab plus apatinib have demonstrated robust antitumor activity and safety in patients with advanced cervical cancer (CLAP study; NCT03816553). We herein present the updated long-term results of the CLAP study and explore potential biomarkers for survival. The outcomes of patients who underwent immune checkpoint inhibitor (ICI) retreatment were also reported.
In this phase II trial, eligible patients received camrelizumab 200 mg intravenously every two weeks and apatinib 250 mg orally once daily in 4-week cycles for up to two years. Treatment was continued until disease progression, unacceptable toxicity, or withdrawal of consent.
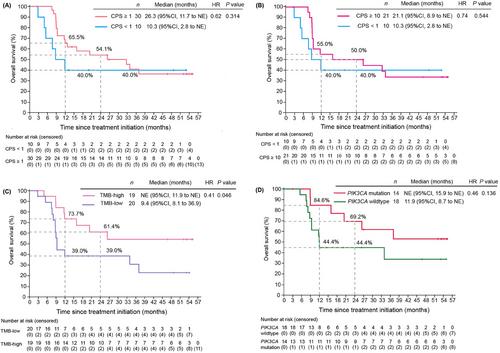
Between January 21 and August 1, 2019, a total of 45 patients were enrolled. Data were analyzed as of July 31, 2023, representing > 48 months since treatment initiation for all patients. Nine (20.0%) patients completed the 2-year study. The median duration of response (DOR) was 16.6 months, and 45.0% of patients achieved a DOR of ≥ 24 months. The 12-month progression-free survival (PFS) rate was 40.7% (95% confidence interval [CI], 25.2-55.6), with an 18-month PFS rate of 37.8% (95% CI, 22.7-52.8). The median overall survival (OS) was 20.3 months (95% CI, 9.3-36.9), and the 24-month OS rate was 47.8% (95% CI, 31.7-62.3). Age > 50 years, programmed death-ligand 1 (PD-L1) combined positive score (CPS) ≥ 1 (versus [vs.] < 1), CPS ≥ 10 (vs. < 1), high tumor mutational burden, and PIK3CA mutations were associated with improved PFS (hazard ratio [HR] < 1) and longer OS (HR < 1). Eight patients who initially responded in the CLAP trial but later experienced disease progression were retreated with ICIs. Among them, 2 (25.0%) achieved a partial response, while 5 (62.5%) had stable disease. Notably, four patients who received retreatment with ICIs survived for more than 45 months. No new safety signals were identified in the present study.
Long-term survival follow-up data demonstrated that camrelizumab plus apatinib has robust, sustained, and durable efficacy in patients with advanced cervical cancer who progress after first-line platinum-based chemotherapy. No new safety signals were noted with long-term treatment.