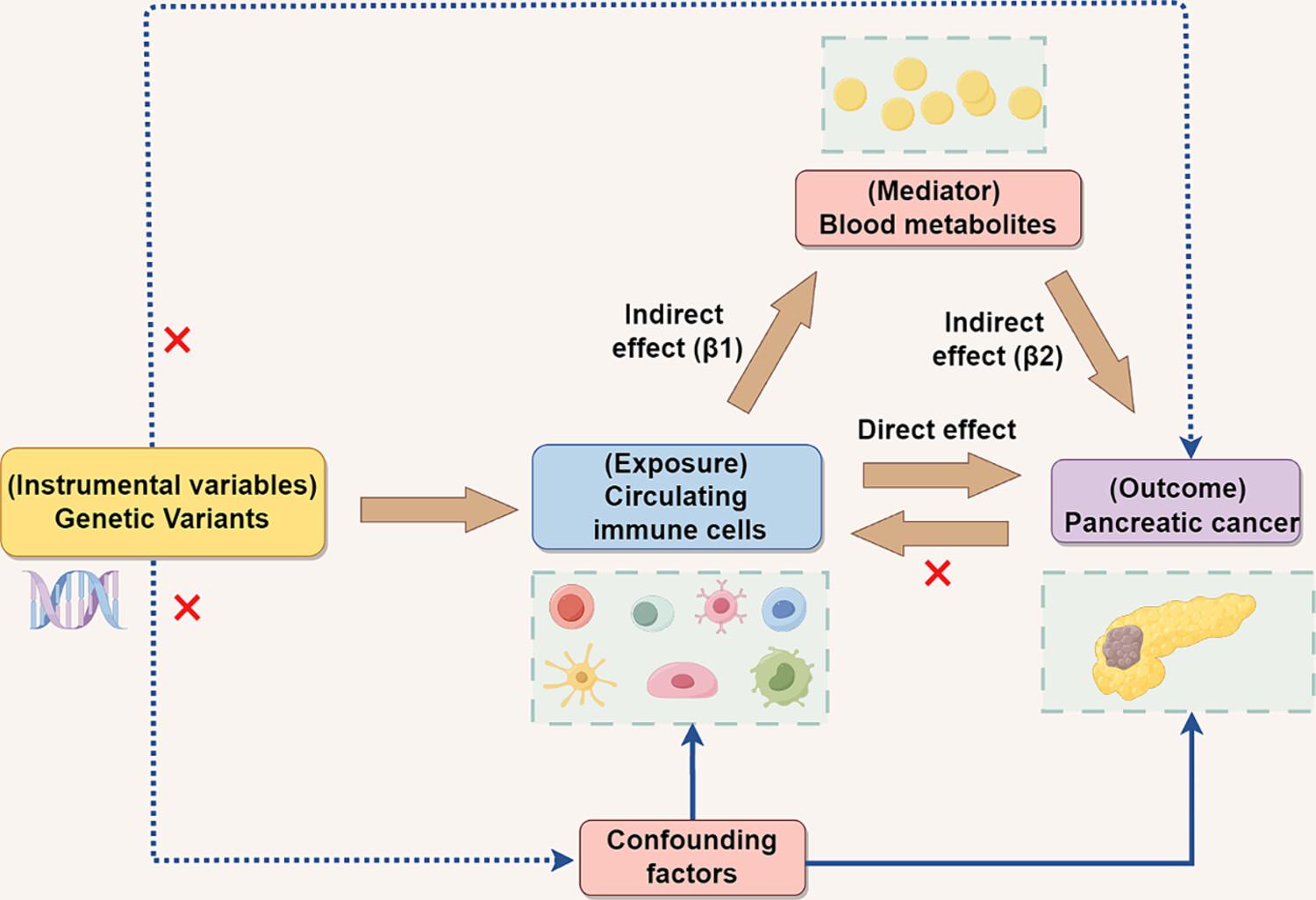
Pancreatic cancer is characterized by metabolic dysregulation and unique immunological profiles. Nevertheless, the comprehensive understanding of immune and metabolic dysregulation of pancreatic cancer remains unclear. In the present study, we aimed to investigate the causal relationship of circulating immune cells and pancreatic cancer and identify the blood metabolites as potential mediators.
The exposure and outcome genome-wide association studies (GWAS) data used in the present study were obtained from the GWAS open-access database (https://gwas.mrcieu.ac.uk). The study used 731 circulating immune cell features, 1400 types of blood metabolites and pancreatic cancer from GWAS. We then performed bidirectional Mendelian randomization (MR) analyses to explore the causal relationships between the circulating immune cells and pancreatic cancer, and two-step MR to discover potential mediating blood metabolites in this process. All statistical analyses were performed in R software. The STROBE-MR (i.e. Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization) checklist for the reporting of MR studies was also used.
MR analysis identified seven types of circulating immune cells causally associated with pancreatic cancer. Furthermore, there was no strong evidence that genetically predicted pancreatic cancer had an effect on these seven types of circulating immune cells. Further two-step MR analysis found 10 types of blood metabolites were causally associated with pancreatic cancer and the associations between circulating CD39+CD8+ T cells and pancreatic cancer were mediated by blood orotates with proportions of 5.18% (p = 0.016).
The present study provides evidence supporting the causal relationships between various circulating immune cells, especially CD39+CD8+ T cells, and pancreatic cancer, with a potential effect mediated by blood orotates. Further research is needed on additional risk factors as potential mediators and establish a comprehensive immunity-metabolism network in pancreatic cancer.