Integrated spatial and multimodal single-cell transcriptomics reveal patient-dependent cell heterogeneity in splenic marginal zone lymphoma
Juan Pablo Cerapio, Pauline Gravelle, Anne Quillet-Mary, Carine Valle, Frederic Martins, Don-Marc Franchini, Charlotte Syrykh, Pierre Brousset, Alexandra Traverse-Glehen, Loic Ysebaert, Jean-Jacques Fournie, Camille Laurent
下载PDF
{"title":"Integrated spatial and multimodal single-cell transcriptomics reveal patient-dependent cell heterogeneity in splenic marginal zone lymphoma","authors":"Juan Pablo Cerapio, Pauline Gravelle, Anne Quillet-Mary, Carine Valle, Frederic Martins, Don-Marc Franchini, Charlotte Syrykh, Pierre Brousset, Alexandra Traverse-Glehen, Loic Ysebaert, Jean-Jacques Fournie, Camille Laurent","doi":"10.1002/path.6296","DOIUrl":null,"url":null,"abstract":"<p>Biological hallmarks of splenic marginal zone lymphoma (SMZL) remain poorly described. Herein, we performed in-depth SMZL characterization through multimodal single-cell analyses of paired blood/spleen samples. The 3’-single-cell RNA-sequencing, Cellular Indexing of Transcriptomes and Epitopes by sequencing, and 5’-V(D)J single-cell RNA-sequencing datasets were integrated to characterize SMZL transcriptome profiles, including B-cell receptor and T-cell receptor repertoires. Hyperexpanded B-cell clones in the spleen were at a memory-like stage, whereas recirculating tumor B-cells in blood encompassed multiple differentiation stages, indicating an unexpected desynchronization of the B-cell maturation program in SMZL cells. Spatial transcriptomics showed the enrichment of T-effector and T-follicular helper (T<sub>FH</sub>) signatures in the nodular subtype of SMZL. This latter also exhibited gene-based cell–cell interactions suggestive of dynamic crosstalk between T<sub>FH</sub> and cancer cells in transcriptomics, further substantiated by using imaging mass cytometry. Our findings provide a comprehensive high-resolution description of SMZL biological hallmarks and characterize, for the first time <i>in situ</i>, inter- and intra-patient heterogeneity at both transcriptomic and protein levels. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 4-5","pages":"442-453"},"PeriodicalIF":5.2000,"publicationDate":"2024-06-03","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6296","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://pathsocjournals.onlinelibrary.wiley.com/doi/10.1002/path.6296","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
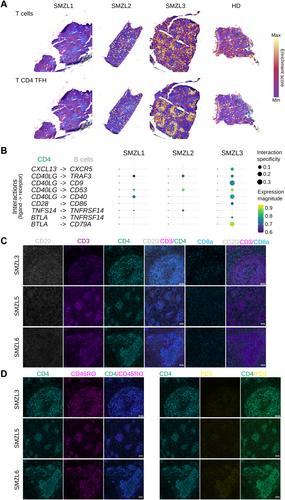
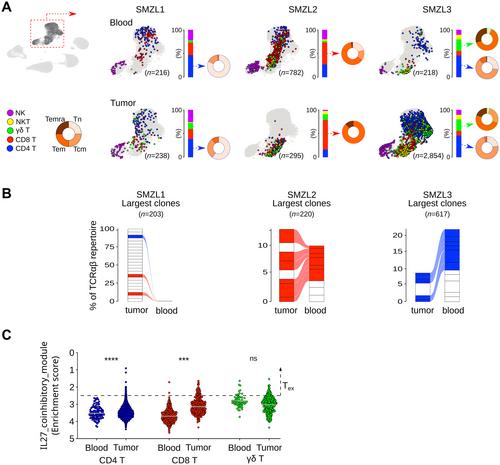
Biological hallmarks of splenic marginal zone lymphoma (SMZL) remain poorly described. Herein, we performed in-depth SMZL characterization through multimodal single-cell analyses of paired blood/spleen samples. The 3’-single-cell RNA-sequencing, Cellular Indexing of Transcriptomes and Epitopes by sequencing, and 5’-V(D)J single-cell RNA-sequencing datasets were integrated to characterize SMZL transcriptome profiles, including B-cell receptor and T-cell receptor repertoires. Hyperexpanded B-cell clones in the spleen were at a memory-like stage, whereas recirculating tumor B-cells in blood encompassed multiple differentiation stages, indicating an unexpected desynchronization of the B-cell maturation program in SMZL cells. Spatial transcriptomics showed the enrichment of T-effector and T-follicular helper (TFH ) signatures in the nodular subtype of SMZL. This latter also exhibited gene-based cell–cell interactions suggestive of dynamic crosstalk between TFH and cancer cells in transcriptomics, further substantiated by using imaging mass cytometry. Our findings provide a comprehensive high-resolution description of SMZL biological hallmarks and characterize, for the first time in situ , inter- and intra-patient heterogeneity at both transcriptomic and protein levels. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
综合空间和多模态单细胞转录组学揭示了脾边缘区淋巴瘤中患者依赖性细胞异质性。
脾脏边缘区淋巴瘤(SMZL)的生物学特征仍然鲜为人知。在此,我们通过对配对的血液/脾脏样本进行多模态单细胞分析,对SMZL的特征进行了深入研究。我们整合了3'-单细胞RNA测序、转录组和表位的细胞索引测序以及5'-V(D)J单细胞RNA测序数据集,以描述SMZL的转录组特征,包括B细胞受体和T细胞受体谱系。脾脏中过度扩增的B细胞克隆处于记忆样阶段,而血液中再循环的肿瘤B细胞则包含多个分化阶段,这表明SMZL细胞中的B细胞成熟程序出现了意想不到的不同步。空间转录组学显示,在SMZL的结节亚型中,T-效应细胞和T-滤泡辅助细胞(TFH)特征丰富。后者还表现出基于基因的细胞-细胞相互作用,表明转录组学中TFH与癌细胞之间存在动态串扰,成像质谱法进一步证实了这一点。我们的研究结果全面、高分辨率地描述了SMZL的生物学特征,并首次在原位从转录组学和蛋白质水平描述了患者之间和患者内部的异质性。© 2024 作者。病理学杂志》由约翰威利父子有限公司代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。