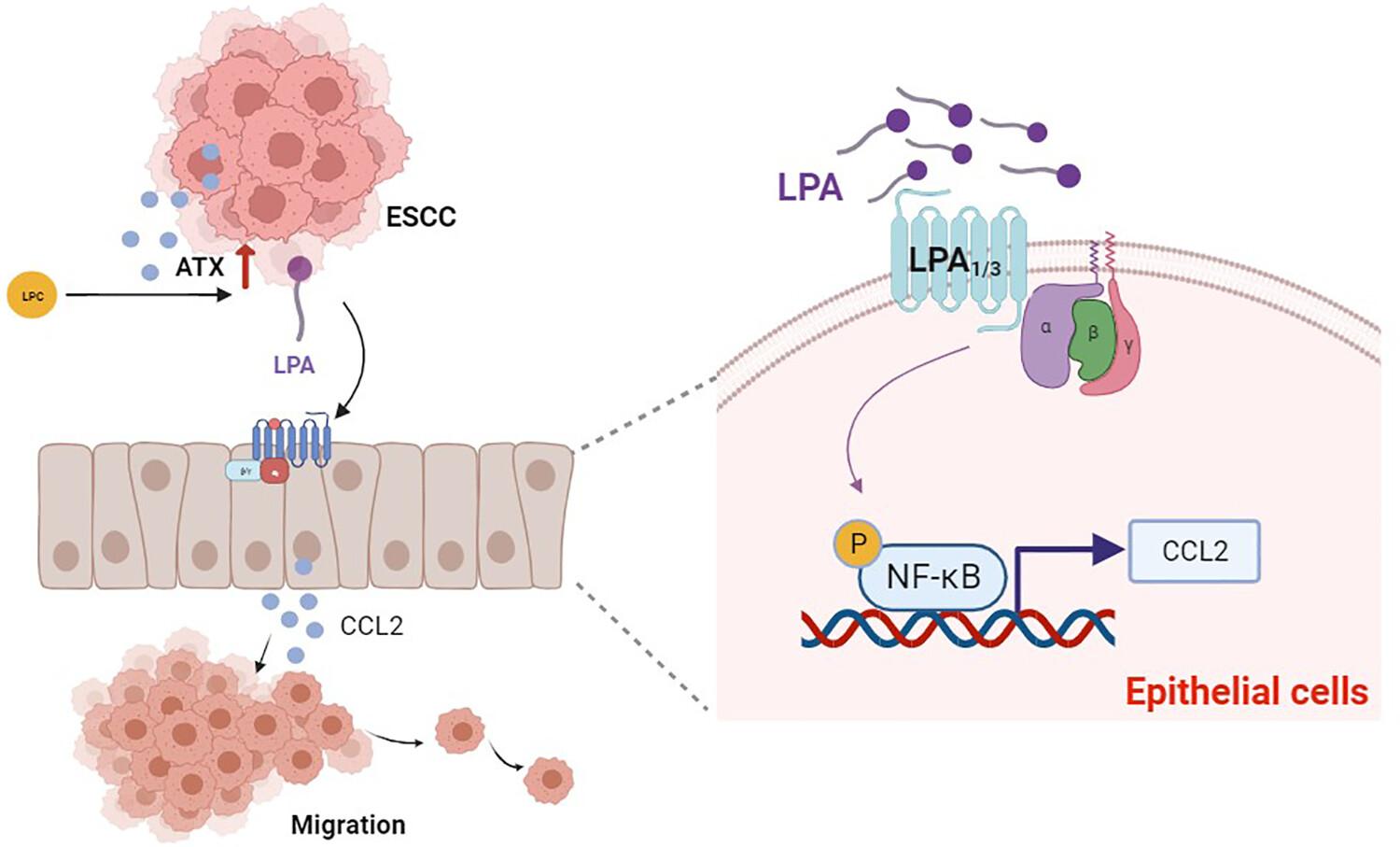
Lysophosphatidic acid (LPA) is a small bioactive lipid which acts as a potent regulator in various tumor progressions through six G-protein-coupled receptors (LPA1–LPA6). Our previous study demonstrated that the LPA-producing enzyme, autotaxin (ATX), was upregulated in esophageal squamous cell carcinoma (ESCC) and ATX high expression levels indicated a poor prognosis. Esophageal squamous cell carcinoma is a type of malignant tumor which originates from epithelial cells. Its progression can be affected by the interaction between cancer cells and normal cells. However, the impact of LPA on the interaction between esophageal epithelial cells and cancer cells in the development of ESCC remains uncertain.
MTS and Edu assays were performed to determine ESCC cell proliferation in culture medium (CM) derived from LPA-stimulated esophageal epithelial cells (Het-1a). A wound healing assay, transwell migration and an invasion assay were performed to assess the metastatic ability of ESCC cells. Cytokine array analysis was conducted to detect the differentially secreted cytokines in CM. The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases were utilized to uncover the pathways and cytokines that are influenced by LPA in ESCC. Immunohistochemical staining was employed to measure the expression of ATX and CCL2 in early-stage ESCC. Quantitative real-time PCR, western blot, enzyme-linked immunosorbent assay and an antibody neutralization assay were employed to measure the mechanism of LPA-mediated communication between epithelial cells and cancer cells.
Functional experiments showed that exposing ESCC cancer cells to CM from LPA-treated Het-1a results in promoting proliferation, migration, invasion and epithelial–mesenchymal transition processes. Using cytokine array analysis, we discovered that LPA triggers the release of multiple cytokines from epithelial cells. After screening of the TCGA and GEO databases, CCL2 was identified and found to be correlated with ATX expression in ESCC. Furthermore, CCL2 levels in both mRNA expression and secretion were observed to be upregulated in epithelial cells upon stimulation with LPA. Blocking CCL2 effectively reduced the pro-migration influence of CM derived from LPA-treated Het-1a. Mechanism studies have demonstrated that LPA activated the NF-κB signaling pathway through LPA1/3, ultimately causing an increase in CCL2 expression and secretion in Het-1a.
Our findings, taken together, demonstrate that CM from LPA-treated esophageal epithelial cells plays a significant role in promoting the progression of ESCC, with CCL2 acting as the primary regulator.