Identification of Ppy-lineage cells as a novel origin of pancreatic ductal adenocarcinoma
Ofejiro Blessing Pereye, Yuko Nakagawa, Takashi Sato, Ayako Fukunaka, Shuhei Aoyama, Yuya Nishida, Wakana Mizutani, Nanami Kobayashi, Yohei Morishita, Tetsunari Oyama, Reika Kawabata-Iwakawa, Hirotaka Watada, Hiroki Mizukami, Akihisa Fukuda, Yoshio Fujitani
下载PDF
{"title":"Identification of Ppy-lineage cells as a novel origin of pancreatic ductal adenocarcinoma","authors":"Ofejiro Blessing Pereye, Yuko Nakagawa, Takashi Sato, Ayako Fukunaka, Shuhei Aoyama, Yuya Nishida, Wakana Mizutani, Nanami Kobayashi, Yohei Morishita, Tetsunari Oyama, Reika Kawabata-Iwakawa, Hirotaka Watada, Hiroki Mizukami, Akihisa Fukuda, Yoshio Fujitani","doi":"10.1002/path.6295","DOIUrl":null,"url":null,"abstract":"<p>The <i>Ppy</i> gene encodes pancreatic polypeptide (PP) secreted by PP- or γ-cells, which are a subtype of endocrine cells localised mainly in the islet periphery. For a detailed characterisation of PP cells, we aimed to establish PP cell lines. To this end, we generated a mouse model harbouring the SV40 large T antigen (TAg) in the <i>Rosa26</i> locus, which is expressed upon <i>Ppy</i>-promoter-mediated Cre–loxP recombination. Whereas <i>Insulin1</i>-<i>Cre</i>ERT-mediated <i>TAg</i> expression in beta cells resulted in insulinoma, surprisingly, <i>Ppy</i>-<i>Cre</i>-mediated <i>TAg</i> expression resulted in the malignant transformation of <i>Ppy</i>-lineage cells. These mice showed distorted islet structural integrity at 5 days of age compared with normal islets. CK19<sup>+</sup> duct-like lesions contiguous with the islets were observed at 2 weeks of age, and mice developed aggressive pancreatic ductal adenocarcinoma (PDAC) at 4 weeks of age, suggesting that PDAC can originate from the islet/endocrine pancreas. This was unexpected as PDAC is believed to originate from the exocrine pancreas. RNA-sequencing analysis of <i>Ppy</i>-lineage islet cells from 7-day-old <i>TAg</i><sup><i>+</i></sup> mice showed a downregulation and an upregulation of endocrine and exocrine genes, respectively, in addition to the upregulation of genes and pathways associated with PDAC. These results suggest that the expression of an oncogene in <i>Ppy</i>-lineage cells induces a switch from endocrine cell fate to PDAC. Our findings demonstrate that <i>Ppy</i>-lineage cells may be an origin of PDAC and may provide novel insights into the pathogenesis of pancreatic cancer, as well as possible therapeutic strategies. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 4-5","pages":"429-441"},"PeriodicalIF":5.6000,"publicationDate":"2024-06-04","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6295","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6295","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
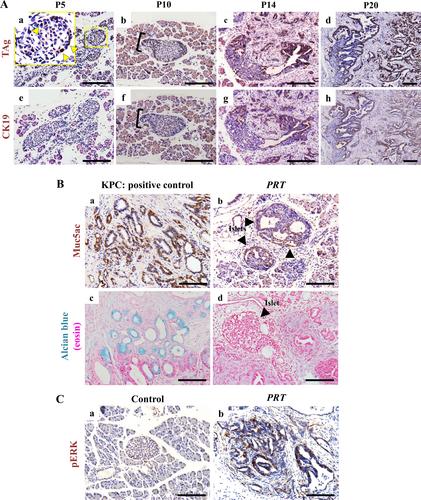
Abstract
The Ppy gene encodes pancreatic polypeptide (PP) secreted by PP- or γ-cells, which are a subtype of endocrine cells localised mainly in the islet periphery. For a detailed characterisation of PP cells, we aimed to establish PP cell lines. To this end, we generated a mouse model harbouring the SV40 large T antigen (TAg) in the Rosa26 locus, which is expressed upon Ppy -promoter-mediated Cre–loxP recombination. Whereas Insulin1 -Cre ERT-mediated TAg expression in beta cells resulted in insulinoma, surprisingly, Ppy -Cre -mediated TAg expression resulted in the malignant transformation of Ppy -lineage cells. These mice showed distorted islet structural integrity at 5 days of age compared with normal islets. CK19+ duct-like lesions contiguous with the islets were observed at 2 weeks of age, and mice developed aggressive pancreatic ductal adenocarcinoma (PDAC) at 4 weeks of age, suggesting that PDAC can originate from the islet/endocrine pancreas. This was unexpected as PDAC is believed to originate from the exocrine pancreas. RNA-sequencing analysis of Ppy -lineage islet cells from 7-day-old TAg + Ppy -lineage cells induces a switch from endocrine cell fate to PDAC. Our findings demonstrate that Ppy -lineage cells may be an origin of PDAC and may provide novel insights into the pathogenesis of pancreatic cancer, as well as possible therapeutic strategies. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.