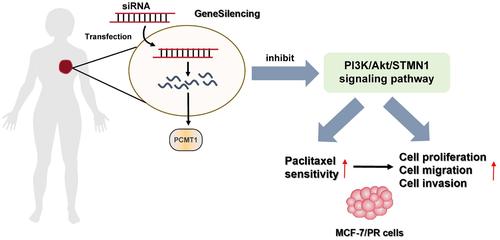
This study aimed to investigate whether silencing Protein L-isoaspartate (D-aspartate) O-methyltransferase (PCMT1) expression can enhance the sensitivity of breast cancer cells to paclitaxel and its possible mechanism. Tumor tissues and adjacent histologically normal tissues were collected from patients with breast cancer admitted to our hospital. Human normal breast epithelial cells MCF10A, human breast cancer cells MCF-7, and paclitaxel-resistant breast cancer cells MCF-7/PR were purchased. MCF-7/PR cells were further grouped into negative control (NC) group, si-PCMT1 group (transfected with si-PCMT1), 740Y-P group (treated with 740Y-P, an activator of phosphatidylinositol 3-kinase (PI3K)/ v-Akt Murine Thymoma Viral Oncogene (AKT) signaling pathway), and si-PCMT1 + 740Y-P group (transfected with si-PCMT1 and then treated with 740Y-P). The expression level of PCMT1 in tissues and cells was detected by quantitative real-time polymerase chain reaction (qRT-PCR). Western blot analysis was used to detect the protein expression level of PCMT1 in tissues and cells as well as the protein level of p-PI3K, PI3K, p-Akt, Akt, and Stathmin1 (STMN1) in cells. 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide (MTT) and colony formation assays were used to determine cell viability, scratch assay was used to assess the migration ability of cells, and Transwell assay was used to assess the invasion ability of cells. The expression of PCMT1 was remarkably up-regulated in breast cancer tissues and MCF-7/PR cells. Silencing PCMT1 expression significantly inhibited the proliferation, migration, and invasion of MCF-7/PR cells, and alleviated the resistance of cancer cells to paclitaxel. Additionally, silencing PCMT1 expression also inhibited the activation of PI3K/Akt/STMN1 pathway in MCF-7/PR cells, while activating PI3K/Akt/STMN1 pathway significantly reversed the effect of silencing PCMT1 expression on MCF-7/PR cells. PCMT1 is highly expressed in breast cancer tissues and MCF-7/PR cells, and silencing PCMT1 expression can not only inhibit the development of breast cancer but also enhance paclitaxel sensitivity. Its mechanism of action may be achieved by inhibiting PI3K/Akt/STMN1 signaling.