Air pollution aggravates renal ischaemia–reperfusion-induced acute kidney injury
Talita Rojas Sanches, Antonio Carlos Parra, Peiqi Sun, Mariana Pereira Graner, Lucas Yuji Umesaki Itto, Loes Maria Butter, Nike Claessen, Joris JTH Roelofs, Sandrine Florquin, Mariana Matera Veras, Maria de Fatima Andrade, Paulo Hilário Nascimento Saldiva, Jesper Kers, Lucia Andrade, Alessandra Tammaro
下载PDF
{"title":"Air pollution aggravates renal ischaemia–reperfusion-induced acute kidney injury","authors":"Talita Rojas Sanches, Antonio Carlos Parra, Peiqi Sun, Mariana Pereira Graner, Lucas Yuji Umesaki Itto, Loes Maria Butter, Nike Claessen, Joris JTH Roelofs, Sandrine Florquin, Mariana Matera Veras, Maria de Fatima Andrade, Paulo Hilário Nascimento Saldiva, Jesper Kers, Lucia Andrade, Alessandra Tammaro","doi":"10.1002/path.6302","DOIUrl":null,"url":null,"abstract":"<p>Chronic kidney disease (CKD) has emerged as a significant global public health concern. Recent epidemiological studies have highlighted the link between exposure to fine particulate matter (PM<sub>2.5</sub>) and a decline in renal function. PM<sub>2.5</sub> exerts harmful effects on various organs through oxidative stress and inflammation. Acute kidney injury (AKI) resulting from ischaemia–reperfusion injury (IRI) involves biological processes similar to those involved in PM<sub>2.5</sub> toxicity and is a known risk factor for CKD. The objective of this study was to investigate the impact of PM<sub>2.5</sub> exposure on IRI-induced AKI. Through a unique environmentally controlled setup, mice were exposed to urban PM<sub>2.5</sub> or filtered air for 12 weeks before IRI followed by euthanasia 48 h after surgery. Animals exposed to PM<sub>2.5</sub> and IRI exhibited reduced glomerular filtration, impaired urine concentration ability, and significant tubular damage. Further, PM<sub>2.5</sub> aggravated local innate immune responses and mitochondrial dysfunction, as well as enhancing cyclic GMP–AMP synthase-stimulator of interferon genes (cGAS–STING) pathway activation. This increased renal senescence and suppressed the anti-ageing protein klotho, leading to early fibrotic changes. <i>In vitro</i> studies using proximal tubular epithelial cells exposed to PM<sub>2.5</sub> and hypoxia/reoxygenation revealed heightened activation of the STING pathway triggered by cytoplasmic mitochondrial DNA, resulting in increased tubular damage and a pro-inflammatory phenotype. In summary, our findings imply a role for PM<sub>2.5</sub> in sensitising proximal tubular epithelial cells to IRI-induced damage, suggesting a plausible association between PM<sub>2.5</sub> exposure and heightened susceptibility to CKD in individuals experiencing AKI. Strategies aimed at reducing PM<sub>2.5</sub> concentrations and implementing preventive measures may improve outcomes for AKI patients and mitigate the progression from AKI to CKD. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 4-5","pages":"496-507"},"PeriodicalIF":5.2000,"publicationDate":"2024-06-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6302","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://pathsocjournals.onlinelibrary.wiley.com/doi/10.1002/path.6302","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
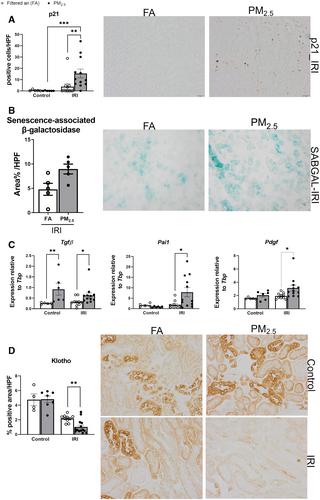
Chronic kidney disease (CKD) has emerged as a significant global public health concern. Recent epidemiological studies have highlighted the link between exposure to fine particulate matter (PM2.5 ) and a decline in renal function. PM2.5 exerts harmful effects on various organs through oxidative stress and inflammation. Acute kidney injury (AKI) resulting from ischaemia–reperfusion injury (IRI) involves biological processes similar to those involved in PM2.5 toxicity and is a known risk factor for CKD. The objective of this study was to investigate the impact of PM2.5 exposure on IRI-induced AKI. Through a unique environmentally controlled setup, mice were exposed to urban PM2.5 or filtered air for 12 weeks before IRI followed by euthanasia 48 h after surgery. Animals exposed to PM2.5 and IRI exhibited reduced glomerular filtration, impaired urine concentration ability, and significant tubular damage. Further, PM2.5 aggravated local innate immune responses and mitochondrial dysfunction, as well as enhancing cyclic GMP–AMP synthase-stimulator of interferon genes (cGAS–STING) pathway activation. This increased renal senescence and suppressed the anti-ageing protein klotho, leading to early fibrotic changes. In vitro studies using proximal tubular epithelial cells exposed to PM2.5 and hypoxia/reoxygenation revealed heightened activation of the STING pathway triggered by cytoplasmic mitochondrial DNA, resulting in increased tubular damage and a pro-inflammatory phenotype. In summary, our findings imply a role for PM2.5 in sensitising proximal tubular epithelial cells to IRI-induced damage, suggesting a plausible association between PM2.5 exposure and heightened susceptibility to CKD in individuals experiencing AKI. Strategies aimed at reducing PM2.5 concentrations and implementing preventive measures may improve outcomes for AKI patients and mitigate the progression from AKI to CKD. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.