Muscle atrophy can cause muscle dysfunction and weakness. Krüppel-like factor 13 (KLF13), a central regulator of cellular energy metabolism, is highly expressed in skeletal muscles and implicated in the pathogenesis of several diseases. This study investigated the role of KLF13 in muscle atrophy, which could be a novel therapeutic target.
The effects of gene knockdown and pharmacological targeting of KLF13 on skeletal muscle atrophy were investigated using cell-based and animal models. Clofoctol, an antibiotic and KLF13 agonist, was also investigated as a candidate for repurposing. The mechanisms related to skeletal muscle atrophy were assessed by measuring the expression levels and activation statuses of key regulatory pathways and validated using gene knockdown and RNA sequencing.
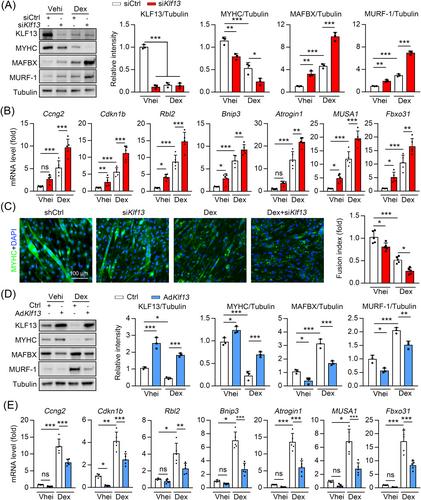
In a dexamethasone-induced muscle atrophy mouse model, the KLF13 knockout group had decreased muscle strength (N) (1.77 ± 0.10 vs. 1.48 ± 0.16, P < 0.01), muscle weight (%) [gastrocnemius (Gas): 76.0 ± 5.69 vs. 60.7 ± 7.23, P < 0.001; tibialis anterior (TA): 75.8 ± 6.21 vs. 67.5 ± 5.01, P < 0.05], and exhaustive running distance (m) (495.5 ± 64.8 vs. 315.5 ± 60.9, P < 0.05) compared with the control group. KLF13 overexpression preserved muscle mass (Gas: 100 ± 6.38 vs. 120 ± 14.4, P < 0.01) and the exhaustive running distance (423.8 ± 59.04 vs. 530.2 ± 77.45, P < 0.05) in an in vivo diabetes-induced skeletal muscle atrophy model. Clofoctol treatment protected against dexamethasone-induced muscle atrophy. Myotubes treated with dexamethasone, an atrophy-inducing glucocorticoid, were aggravated by KLF13 knockout, but anti-atrophic effects were achieved by inducing KLF13 overexpression. We performed a transcriptome analysis and luciferase reporter assays to further explore this mechanism, finding that delta-like 4 (Dll4) was a novel target gene of KLF13. The KLF13 transcript repressed Dll4, inhibiting the Dll4-Notch2 axis and preventing muscle atrophy. Dexamethasone inhibited KLF13 expression by inhibiting myogenic differentiation 1 (i.e., MYOD1)-mediated KLF13 transcriptional activation and promoting F-Box and WD repeat domain containing 7 (i.e., FBXW7)-mediated KLF13 ubiquitination.
This study sheds new light on the mechanisms underlying skeletal muscle atrophy and potential drug targets. KLF13 regulates muscle atrophy and is a potential therapeutic target. Clofoctol is an attractive compound for repurposing studies to treat skeletal muscle atrophy.