The comparative effectiveness of selective serotonin reuptake inhibitors (SSRIs) has been subjected to relatively little research. However, a recent study based on target trial emulation suggested that sertraline may be more effective than escitalopram.
To investigate whether sertraline, citalopram, and escitalopram differ in their effectiveness—assessed via the risk of psychiatric hospital admission and suicide following treatment initiation. The choice to focus on sertraline, citalopram, and escitalopram was made to limit confounding by indication, as the Danish depression treatment guideline from 2007 specifically listed these three SSRIs as first choice.
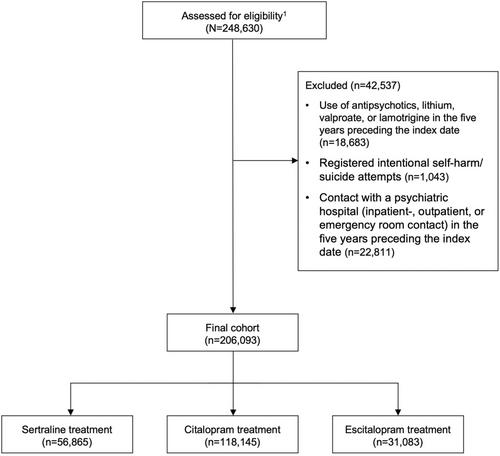
We conducted a target trial emulation based on data from Danish registers. We identified all individuals that initiated treatment for depression with sertraline, citalopram, or escitalopram in the period from January 1, 2007, to March 1, 2019. These individuals were followed until psychiatric hospital admission or suicide (separate analyses), death, 1 year after treatment initiation or end of data. Cox proportional hazards regression adjusted for relevant baseline covariates was performed to emulate randomized treatment allocation, comparing the rate of psychiatric hospital admission and suicide for individuals treated with sertraline (used as reference), citalopram or escitalopram, respectively. For escitalopram, we conducted a sensitivity analysis excluding data from the period during which the drug was sold under patent, as the price of the drug during that time likely entailed a different prescription pattern, increasing the risk of (“patent-related”) confounding by indication.
We identified 56,865, 118,145, and 31,083 individuals initiating treatment with sertraline, citalopram, and escitalopram, respectively. Using sertraline as reference, the adjusted hazard rate ratio (aHRR) for psychiatric admission was 0.98 (95% CI = 0.91–1.05) for citalopram and 1.21 (95% CI = 1.10–1.32) for escitalopram. Notably, in the sensitivity analysis only including patients initiating treatment after the escitalopram patent had expired, the increased risk of psychiatric hospital admission associated with escitalopram treatment was no longer present (aHRR = 0.98, 95% CI = 0.82–1.18). The results of the analyses of suicide were inconclusive, due to few outcome events.
Sertraline, citalopram, and escitalopram do not seem to have differential effectiveness in the treatment of depression. Taking potential patent-related, time varying, confounding by indication (via severity) into account is critical for pharmacoepidemiological studies, including those employing target trial emulation.