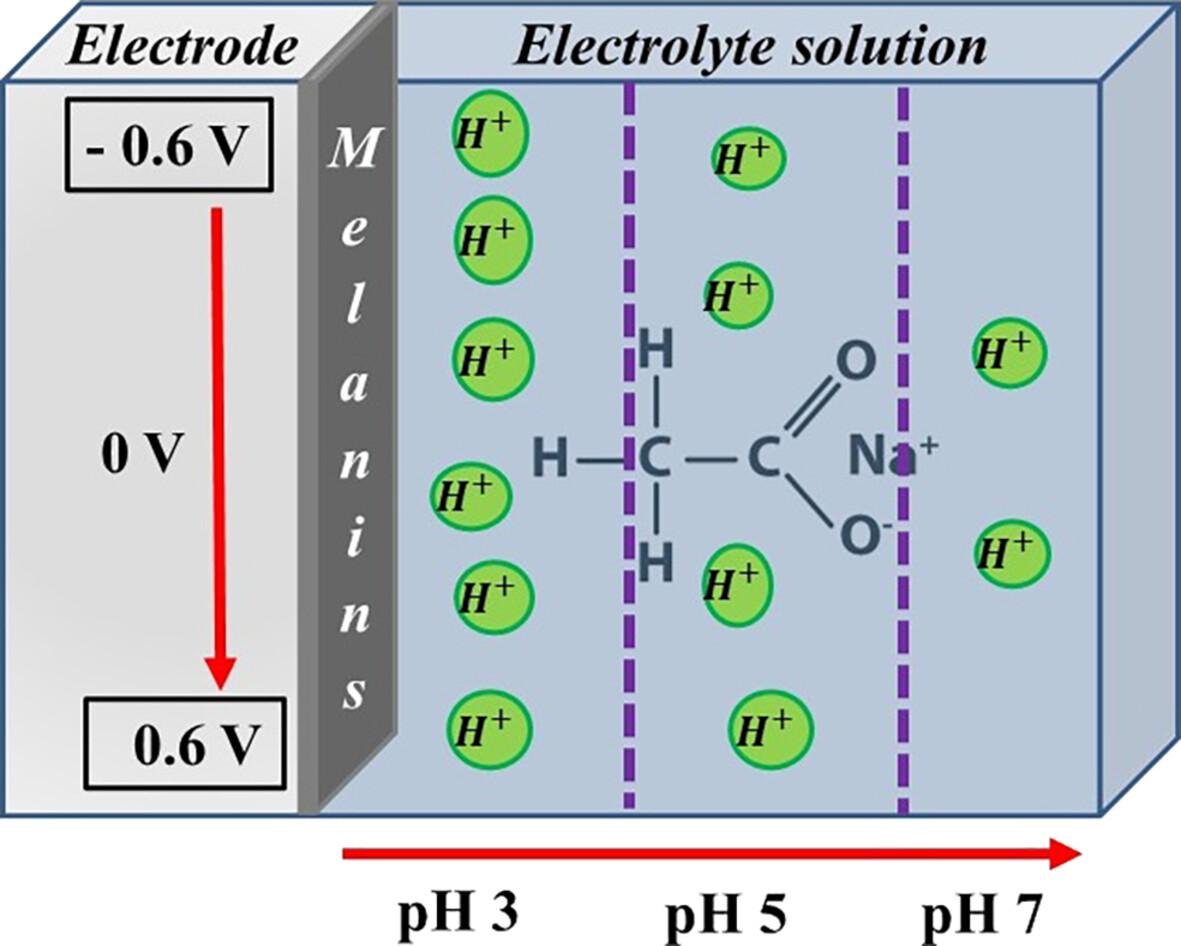
Electrochemical behavior of non-functionalized and sulfonated melanins at different pH values
Nayrim Brizuela Guerra, João Victor Morais Lima, João Vitor Paulin, Natan Luiz Nozella, Miguel Henrique Boratto, Gabriel Leonardo Nogueira, Carlos César Bof Bufon, Carlos Frederico de Oliveira Graeff
求助PDF
{"title":"Electrochemical behavior of non-functionalized and sulfonated melanins at different pH values","authors":"Nayrim Brizuela Guerra, João Victor Morais Lima, João Vitor Paulin, Natan Luiz Nozella, Miguel Henrique Boratto, Gabriel Leonardo Nogueira, Carlos César Bof Bufon, Carlos Frederico de Oliveira Graeff","doi":"10.1002/pi.6678","DOIUrl":null,"url":null,"abstract":"<p>Melanins are macromolecular pigments widely spread in many living organisms, with unique physical and chemical properties. Specifically, their conductive properties have drawn attention for applications in different devices; however, the electrochemical response is equally necessary for designing technologies in sustainable bioelectronics. In this sense, we report a comparative study of the redox electrochemical properties of non-functionalized and sulfonated melanins in different pH environments. The electrochemical response was investigated using cyclic voltammetry, electrochemical impedance spectroscopy, dielectric permittivity and AC/DC conductivity at pH 3, 5 and 7. The voltammetric currents were higher at low pH, in agreement with the known proton transport properties of melanins. The effect of pH on electrochemical properties was slightly more significant in non-functionalized pigments. Melanins with a higher 5,6-dihydroxyindole carboxylic acid/5,6-dihydroxyindole ratio showed high DC current and low impedance. No significant difference was observed in the dielectric relaxation process between the different samples. © 2024 Society of Chemical Industry.</p>","PeriodicalId":20404,"journal":{"name":"Polymer International","volume":"73 11","pages":"992-1000"},"PeriodicalIF":2.9000,"publicationDate":"2024-07-12","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Polymer International","FirstCategoryId":"92","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/pi.6678","RegionNum":4,"RegionCategory":"化学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"POLYMER SCIENCE","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
Melanins are macromolecular pigments widely spread in many living organisms, with unique physical and chemical properties. Specifically, their conductive properties have drawn attention for applications in different devices; however, the electrochemical response is equally necessary for designing technologies in sustainable bioelectronics. In this sense, we report a comparative study of the redox electrochemical properties of non-functionalized and sulfonated melanins in different pH environments. The electrochemical response was investigated using cyclic voltammetry, electrochemical impedance spectroscopy, dielectric permittivity and AC/DC conductivity at pH 3, 5 and 7. The voltammetric currents were higher at low pH, in agreement with the known proton transport properties of melanins. The effect of pH on electrochemical properties was slightly more significant in non-functionalized pigments. Melanins with a higher 5,6-dihydroxyindole carboxylic acid/5,6-dihydroxyindole ratio showed high DC current and low impedance. No significant difference was observed in the dielectric relaxation process between the different samples. © 2024 Society of Chemical Industry.
非官能化和磺化黑色素在不同 pH 值下的电化学行为
黑色素是广泛存在于许多生物体内的大分子色素,具有独特的物理和化学特性。具体来说,它们的导电性能引起了人们对其在不同设备中应用的关注;然而,电化学反应对于设计可持续生物电子学技术也同样必要。因此,我们报告了一项关于非官能化黑色素和磺化黑色素在不同 pH 值环境下的氧化还原电化学性质的比较研究。我们采用循环伏安法、电化学阻抗光谱法、介电常数法和交直流电导法研究了黑色素在 pH 值为 3、5 和 7 时的电化学反应。在 pH 值较低时,伏安电流较高,这与已知的黑色素质子传输特性一致。在非官能化色素中,pH 值对电化学特性的影响略微显著。5,6-二羟基吲哚羧酸/5,6-二羟基吲哚比率较高的黑色素显示出较高的直流电流和较低的阻抗。不同样品的介电弛豫过程没有明显差异。© 2024 化学工业协会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。