Intensive care unit-acquired weakness (ICU-AW) is a syndrome characterized by a long-term muscle weakness often observed in sepsis-surviving patients during the chronic phase. Although ICU-AW is independently associated with increased mortality, effective therapies have yet to be established. Programmed death-1 (PD-1) inhibitors have attracted attention as potential treatments for reversing immune exhaustion in sepsis; however, its impact on ICU-AW remains to be elucidated. Here, we study how PD-1 deficiency affects sepsis-induced skeletal muscle dysfunction in a preclinical sepsis model.
Chronic sepsis model was developed by treating wild-type (WT) and PD-1 knockout (KO) mice with caecal slurry, followed by resuscitation with antibiotics and saline. Mice were euthanized on days 15–17. Body weights, muscle weights, and limb muscle strengths were measured. Interleukin 13 (IL-13) and PD-1 expressions were examined by flow cytometry. Messenger RNA (mRNA) expressions of slow-twitch muscles were measured by reverse transcription and quantitative polymerase chain reaction (RT-qPCR). In an in vitro study, C2C12 myotubes were treated with lipopolysaccharide (LPS) and recombinant IL-13 followed by gene expression measurements.
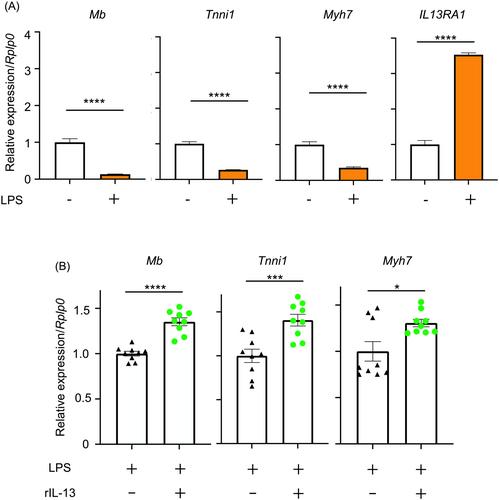
WT septic mice exhibited decreased muscle weight (quadriceps, P < 0.01; gastrocnemius, P < 0.05; and tibialis anterior, P < 0.01) and long-term muscle weakness (P < 0.0001), whereas PD-1 KO septic mice did not exhibit any reduction in muscle weights and strengths. Slow-twitch specific mRNAs, including myoglobin (Mb), troponin I type 1 (Tnni1), and myosin heavy chain 7 (Myh7) were decreased in WT skeletal muscle (Mb, P < 0.0001; Tnni1, P < 0.05; and Myh7, P < 0.05) after sepsis induction, but mRNA expressions of Tnni1 and Myh7 were increased in PD-1 KO septic mice (Mb, not significant; Tnni1, P < 0.0001; and Myh7, P < 0.05). Treatment of C2C12 myotube cells with LPS decreased the expression of slow-twitch mRNAs, which was restored by IL-13 (Mb, P < 0.0001; Tnni1, P < 0.001; and Myh7, P < 0.05). IL-13 production was significantly higher in ILC2s compared to T cells in skeletal muscle (P < 0.05). IL-13-producing ILC2s in skeletal muscle were examined and found to increase in PD-1 KO septic mice, compared with WT septic mice (P < 0.05). ILC2-derived IL-13 was increased by PD-1 KO septic mice and thought to protect the muscles from experimental ICU-AW.
Long-term muscle weakness in experimental ICU-AW was ameliorated in PD-1 KO mice. ILC2-derived IL-13 production in skeletal muscles was increased in PD-1 KO mice, thereby suggesting that IL-13 alleviates muscle weakness during sepsis. This study demonstrates the effects of PD-1 blockade in preserving muscle strength during sepsis through an increase in ILC2-derived IL-13 and may be an attractive therapeutic target for sepsis-induced ICU-AW.