Expanded Antigen-Specific Elimination Assay to Measure Human CD8+ T Cell Cytolytic Potential
David R. Collins, Mpho J. Olatotse, Zachary J. Racenet, Umar Arshad, Elif Çakan, Gaurav D. Gaiha, Kiera L. Clayton, Bruce D. Walker
{"title":"Expanded Antigen-Specific Elimination Assay to Measure Human CD8+ T Cell Cytolytic Potential","authors":"David R. Collins, Mpho J. Olatotse, Zachary J. Racenet, Umar Arshad, Elif Çakan, Gaurav D. Gaiha, Kiera L. Clayton, Bruce D. Walker","doi":"10.1002/cpz1.1109","DOIUrl":null,"url":null,"abstract":"<p>Durable cellular immunity against pathogens is dependent upon a coordinated recall response to antigen by memory CD8<sup>+</sup> T cells, involving their proliferation and the generation of secondary cytotoxic effector cells. Conventional assays measuring <i>ex vivo</i> cytotoxicity fail to capture this secondary cytolytic potential, especially in settings where cells have not been recently exposed to their cognate antigen <i>in vivo</i>. Here we describe the expanded antigen-specific elimination assay (EASEA), a flow cytometric endpoint assay to measure the capacity of human CD8<sup>+</sup> T cells to expand <i>in vitro</i> upon antigen re-exposure and generate secondary effector cells capable of selectively eliminating autologous antigen-pulsed target cells across a range of effector-to-target ratios. Unlike alternative assays, EASEA avoids the hazards of radioactive labeling and viral infection and can be used to study responses to individual or pooled antigens of interest. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol</b>: Expanded antigen-specific elimination assay</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 7","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2024-07-18","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1109","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.1109","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
Durable cellular immunity against pathogens is dependent upon a coordinated recall response to antigen by memory CD8+ T cells, involving their proliferation and the generation of secondary cytotoxic effector cells. Conventional assays measuring ex vivo cytotoxicity fail to capture this secondary cytolytic potential, especially in settings where cells have not been recently exposed to their cognate antigen in vivo. Here we describe the expanded antigen-specific elimination assay (EASEA), a flow cytometric endpoint assay to measure the capacity of human CD8+ T cells to expand in vitro upon antigen re-exposure and generate secondary effector cells capable of selectively eliminating autologous antigen-pulsed target cells across a range of effector-to-target ratios. Unlike alternative assays, EASEA avoids the hazards of radioactive labeling and viral infection and can be used to study responses to individual or pooled antigens of interest. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol: Expanded antigen-specific elimination assay

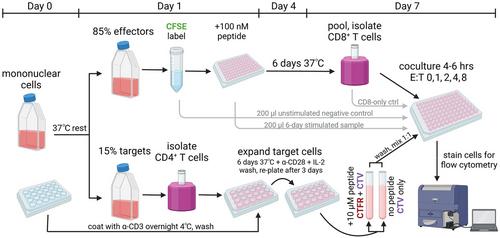
用于测量人类 CD8+ T 细胞细胞溶解潜能的扩展抗原特异性消除试验
针对病原体的持久细胞免疫依赖于记忆 CD8+ T 细胞对抗原的协调回忆反应,包括细胞增殖和产生次级细胞毒性效应细胞。测量体内外细胞毒性的传统检测方法无法捕捉到这种次级细胞溶解潜能,尤其是在细胞最近未在体内暴露于其同源抗原的情况下。在这里,我们介绍了扩增抗原特异性消除测定(EASEA),这是一种流式细胞计数终点测定法,用于测量人类 CD8+ T 细胞在体外扩增后再次暴露于抗原并产生次级效应细胞的能力,这些细胞能够在效应细胞与靶细胞的比例范围内选择性地消除自体抗原脉冲靶细胞。与其他检测方法不同,EASEA 避免了放射性标记和病毒感染的危害,可用于研究对单个或集合抗原的反应。© 2024 作者。当前协议》由 Wiley Periodicals LLC 出版。基本方案:扩大抗原特异性消除试验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。



