{"title":"Generation and Characterization of hiPSC-Derived Vascularized-, Perfusable Cardiac Microtissues-on-Chip","authors":"Ulgu Arslan, Francijna E. van den Hil, Christine L. Mummery, Valeria Orlova","doi":"10.1002/cpz1.1097","DOIUrl":null,"url":null,"abstract":"<p>In the heart <i>in vivo</i>, vasculature forms a semi-permeable endothelial barrier for selective nutrient and (immune) cell delivery to the myocardium and removal of waste products. Crosstalk between the vasculature and the heart cells regulates homeostasis in health and disease. To model heart development and disease <i>in vitro</i> it is important that essential features of this crosstalk are captured. Cardiac organoid and microtissue models often integrate endothelial cells (ECs) to form microvascular networks inside the 3D structure. However, in static culture without perfusion, these networks may fail to show essential functionality. Here, we describe a protocol to generate an <i>in vitro</i> model of human induced pluripotent stem cell (hiPSC)-derived vascularized cardiac microtissues on a microfluidic organ-on-chip platform (VMToC) in which the blood vessels are perfusable. First, prevascularized cardiac microtissues (MT) are formed by combining hiPSC-derived cardiomyocytes, ECs, and cardiac fibroblasts in a pre-defined ratio. Next, these prevascularized MTs are integrated in the chips in a fibrin hydrogel containing additional vascular cells, which self-organize into tubular structures. The MTs become vascularized through anastomosis between the pre-existing microvasculature in the MT and the external vascular network. The VMToCs are then ready for downstream structural and functional assays and basic characterization. Using this protocol, cardiac MTs can be efficiently and robustly vascularized and perfused within 7 days. <i>In vitro</i> vascularized organoid and MT models have the potential to transition current 3D cardiac models to more physiologically relevant organ models that allow the role of the endothelial barrier in drug and inflammatory response to be investigated. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol</b>: Generation of VMToC</p><p><b>Support Protocol 1</b>: Functional Characterization of VMToC</p><p><b>Support Protocol 2</b>: Structural Characterization of VMToC</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 7","pages":""},"PeriodicalIF":0.0000,"publicationDate":"2024-07-22","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1097","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/cpz1.1097","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
In the heart in vivo, vasculature forms a semi-permeable endothelial barrier for selective nutrient and (immune) cell delivery to the myocardium and removal of waste products. Crosstalk between the vasculature and the heart cells regulates homeostasis in health and disease. To model heart development and disease in vitro it is important that essential features of this crosstalk are captured. Cardiac organoid and microtissue models often integrate endothelial cells (ECs) to form microvascular networks inside the 3D structure. However, in static culture without perfusion, these networks may fail to show essential functionality. Here, we describe a protocol to generate an in vitro model of human induced pluripotent stem cell (hiPSC)-derived vascularized cardiac microtissues on a microfluidic organ-on-chip platform (VMToC) in which the blood vessels are perfusable. First, prevascularized cardiac microtissues (MT) are formed by combining hiPSC-derived cardiomyocytes, ECs, and cardiac fibroblasts in a pre-defined ratio. Next, these prevascularized MTs are integrated in the chips in a fibrin hydrogel containing additional vascular cells, which self-organize into tubular structures. The MTs become vascularized through anastomosis between the pre-existing microvasculature in the MT and the external vascular network. The VMToCs are then ready for downstream structural and functional assays and basic characterization. Using this protocol, cardiac MTs can be efficiently and robustly vascularized and perfused within 7 days. In vitro vascularized organoid and MT models have the potential to transition current 3D cardiac models to more physiologically relevant organ models that allow the role of the endothelial barrier in drug and inflammatory response to be investigated. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol: Generation of VMToC
Support Protocol 1: Functional Characterization of VMToC
Support Protocol 2: Structural Characterization of VMToC
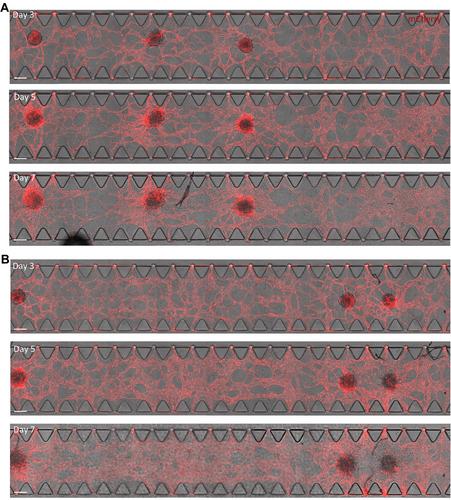
hiPSC 衍生的血管化、可灌注心脏芯片微组织的生成与特征描述。
在活体心脏中,血管形成了一个半渗透性的内皮屏障,有选择性地向心肌输送营养物质和(免疫)细胞,并清除废物。血管和心脏细胞之间的相互作用调节着健康和疾病的平衡。要在体外建立心脏发育和疾病模型,就必须捕捉到这种串扰的基本特征。心脏器官模型和微组织模型通常整合内皮细胞(EC),在三维结构内形成微血管网络。然而,在没有灌注的静态培养中,这些网络可能无法显示基本功能。在这里,我们描述了一种在微流控芯片器官平台(VMToC)上生成人类诱导多能干细胞(hiPSC)衍生的血管化心脏微组织体外模型的方案,其中的血管是可灌注的。首先,将源自 hiPSC 的心肌细胞、EC 和心脏成纤维细胞按预先确定的比例组合在一起,形成血管前心脏微组织(MT)。然后,将这些血管化前的 MT 整合到含有额外血管细胞的纤维蛋白水凝胶芯片中,使其自我组织成管状结构。通过 MT 中预先存在的微血管与外部血管网络之间的吻合,MT 实现血管化。然后,VMToCs 就可以进行下游结构和功能测试以及基本表征了。采用这种方案,心脏 MT 可在 7 天内高效、稳健地血管化和灌注。体外血管化的类器官和MT模型有可能将目前的三维心脏模型转变为更贴近生理的器官模型,从而可以研究内皮屏障在药物和炎症反应中的作用。© 2024 作者简介当前协议》由 Wiley Periodicals LLC 出版。基本协议:支持协议 1:VMToC 的功能表征 支持协议 2:VMToC 的结构表征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。


