Clinical recognition of potential immune-mediated allergic responses to implanted metal devices is increasing. For orthopaedic implants, while ‘pure’ compounds are used in specific circumstances, the majority of components are alloys – a combination of two or more distinct metals. Titanium is found commonly in many orthopaedic devices and is often championed as a ‘hypoallergenic’ option or inclusion. In the absence of a relevant previously published summary on the topic, this paper explores the current state-of-understanding of titanium allergy and proposes a patient management algorithm whereby such immune reactions are clinically-suggested.
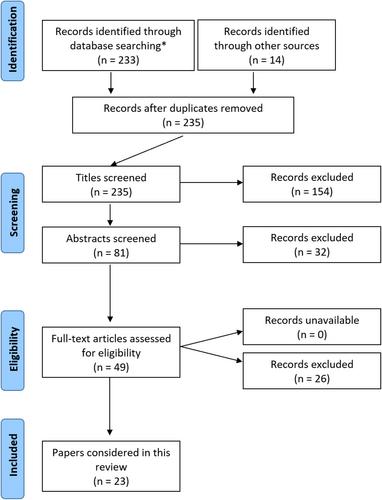
A structured, systematic literature review was performed following PRISMA search principles to provide a contemporary summary-of-understanding in this area and to highlight clinical and knowledge deficiencies.
Thirty-five topic-related articles were identified, the majority reflecting small case series' or proof-of-concept studies. The general standard of scientific evidence available was poor. Justification for arthroplasty utilization of titanium as a ‘hypoallergenic’ option is largely extrapolated from non-orthopaedic domains.
Both ionic and conjugated titanium particles released from implant surfaces have the potential to trigger innate immune responses and true allergy. There exists no simple, high-sensitivity, screening test for titanium allergy. Conventional skin-patch testing is unreliable due to poor dermal penetration. Given established lymphocyte and macrophage activation pathways for allergy responses, in vitro methods using both cell-types show diagnostic promise. Surgical biopsy analysis from host-implant interfaces remains the contemporary ‘gold-standard’, however this represents an invasive, costly and highly-specialized approach not readily available in most settings. Further research to establish reliable/accessible diagnostic methods are indicated.