Chronic obstructive pulmonary disease (COPD) is a heterogeneous progressive lung condition characterized by long-term respiratory symptoms and airflow limitation. Appropriate bronchodilation is the cornerstone of COPD treatment, leading to better health status as well as benefits in prognosis and mortality.
In the current open, noninterventional, observational study, 716 patients diagnosed with COPD of variable severity were administered a fixed-dose combination (FDC) of fluticasone propionate and salmeterol (500 + 50 mcg) through the Elpenhaler® device. The patients' adherence to treatment (based on the MMAS-8 [8-item Morisky Medication Adherence Scale]) and health status (based on the CCQ [Clinical COPD Questionnaire]) were assessed at the beginning of the study and at the end of the 3-month follow-up period.
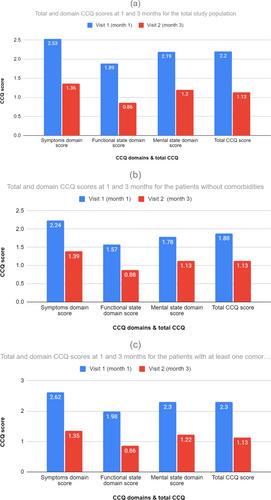
The mean ± SD MMAS-8 score at 1 and 3 months was 6.12 ± 1.89 and 6.45 ± 1.80, respectively, indicating medium adherence overall; however, there was a statistically significant increase of 0.33 units in the MMAS-8 score at the end of the follow-up (paired t-test p < 0.0001), suggestive of an improvement in adherence throughout the study. Higher adherence was associated with better health status at baseline, which further improved by the end of the follow-up. Moreover, we observed a statistically significant decrease of 1.07 points (p < 0.0001) in the mean CCQ total score from the baseline (CCQ score = 2.2 ± 1.00) until the end of the study follow-up (CCQ score = 1.13 ± 0.67). Similar conclusions were also drawn in the mean domain scores regarding symptoms (score equal to 1.36 ± 0.72, decrease by 1.18) as well as functional and mental state (scores equal to 0.86 ± 0.73 and 1.20 ± 0.88, decrease by 1.04 and 1.00, respectively, p < 0.0001). Similarly, when patients were stratified into subgroups with and without comorbidities, the former group showed an increase of 7% in the patients with medium to high adherence during the course of the study. In the same patient subgroup, there was a notable decrease in CCQ score by 1.18 points (p < 0.0001) during the study.
The administration of FDC of fluticasone propionate and salmeterol, (500 + 50 mcg) via the Elpenhaler® device for COPD, resulted in a well-maintained or slight increase in treatment adherence and a subsequent benefit in health status, which further persisted after 3 months of treatment.