{"title":"Defining PANoptosis: Biochemical and Mechanistic Evaluation of Innate Immune Cell Death Activation","authors":"Rebecca E. Tweedell, Taylor Hibler, Thirumala-Devi Kanneganti","doi":"10.1002/cpz1.1112","DOIUrl":null,"url":null,"abstract":"<p>The innate immune system is the first line of host defense. Innate immune activation utilizes pattern recognition receptors to detect pathogens, pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs), and homeostatic alterations and drives inflammatory signaling pathways and regulated cell death. Cell death activation is critical to eliminate pathogens and aberrant or damaged cells, while excess activation can be linked to inflammation, tissue damage, and disease. Therefore, there is increasing interest in studying cell death mechanisms to understand the underlying biology and identify therapeutic strategies. However, there are significant technical challenges, as many cell death pathways share key molecules with each other, and genetic models where these cell death molecules are deleted remain the gold standard for evaluation. Furthermore, extensive crosstalk has been identified between the cell death pathways pyroptosis, apoptosis, necroptosis, and the more recently characterized PANoptosis, which is defined as a prominent, unique innate immune, lytic, and inflammatory cell death pathway initiated by innate immune sensors and driven by caspases and RIPKs through PANoptosomes. PANoptosomes are multi-protein complexes assembled by innate immune sensor(s) in response to pathogens, PAMPs, DAMPs, cytokines, and homeostatic changes that drive PANoptosis. In this article, we provide methods for molecularly defining distinct cell death pathways, including PANoptosis, using both genetic and chemical approaches through western blot, LDH assay, and microscopy readouts. This procedure allows for the assessment of cell death on the cell population and single-cell levels even without access to genetic models. Having this comprehensive workflow that is more accessible to all labs will improve our ability as a scientific community to accelerate discovery. Using these protocols will help identify new innate immune sensors that drive PANoptosis and define the molecular mechanisms and regulators involved to establish new targets for clinical translation. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Induction and quantification of cell death using live cell imaging</p><p><b>Alternate Protocol 1</b>: Quantification of cell death using LDH</p><p><b>Alternate Protocol 2</b>: Assessment of cell death complexes in single cells using immunofluorescence staining</p><p><b>Basic Protocol 2</b>: Analysis of cell death mechanisms by immunoblots (western blots)</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 7","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2024-07-29","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1112","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.1112","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
The innate immune system is the first line of host defense. Innate immune activation utilizes pattern recognition receptors to detect pathogens, pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs), and homeostatic alterations and drives inflammatory signaling pathways and regulated cell death. Cell death activation is critical to eliminate pathogens and aberrant or damaged cells, while excess activation can be linked to inflammation, tissue damage, and disease. Therefore, there is increasing interest in studying cell death mechanisms to understand the underlying biology and identify therapeutic strategies. However, there are significant technical challenges, as many cell death pathways share key molecules with each other, and genetic models where these cell death molecules are deleted remain the gold standard for evaluation. Furthermore, extensive crosstalk has been identified between the cell death pathways pyroptosis, apoptosis, necroptosis, and the more recently characterized PANoptosis, which is defined as a prominent, unique innate immune, lytic, and inflammatory cell death pathway initiated by innate immune sensors and driven by caspases and RIPKs through PANoptosomes. PANoptosomes are multi-protein complexes assembled by innate immune sensor(s) in response to pathogens, PAMPs, DAMPs, cytokines, and homeostatic changes that drive PANoptosis. In this article, we provide methods for molecularly defining distinct cell death pathways, including PANoptosis, using both genetic and chemical approaches through western blot, LDH assay, and microscopy readouts. This procedure allows for the assessment of cell death on the cell population and single-cell levels even without access to genetic models. Having this comprehensive workflow that is more accessible to all labs will improve our ability as a scientific community to accelerate discovery. Using these protocols will help identify new innate immune sensors that drive PANoptosis and define the molecular mechanisms and regulators involved to establish new targets for clinical translation. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Induction and quantification of cell death using live cell imaging
Alternate Protocol 1: Quantification of cell death using LDH
Alternate Protocol 2: Assessment of cell death complexes in single cells using immunofluorescence staining
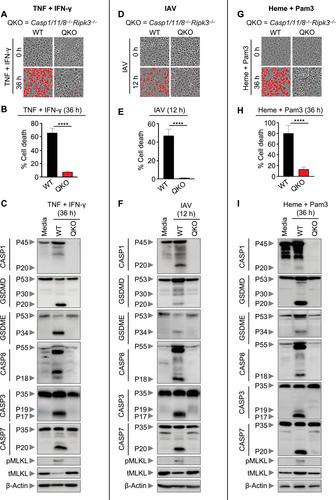
Basic Protocol 2: Analysis of cell death mechanisms by immunoblots (western blots)

定义泛凋亡:先天性免疫细胞死亡激活的生化和机制评估。
先天性免疫系统是宿主防御的第一道防线。先天性免疫激活利用模式识别受体来检测病原体、病原体相关分子模式和损伤相关分子模式(PAMPs 和 DAMPs)以及体内平衡的改变,并驱动炎症信号通路和调节细胞死亡。细胞死亡激活对于消除病原体和异常或受损细胞至关重要,而过度激活则可能与炎症、组织损伤和疾病有关。因此,人们对细胞死亡机制的研究兴趣与日俱增,以了解潜在的生物学原理并确定治疗策略。然而,由于许多细胞死亡途径彼此共享关键分子,因此存在重大的技术挑战,而删除这些细胞死亡分子的遗传模型仍然是评估的黄金标准。此外,已发现细胞死亡途径热凋亡、细胞凋亡、坏死和最近表征的 PANoptosis 之间存在广泛的串扰,PANoptosis 被定义为一种突出的、独特的先天性免疫、溶解和炎症细胞死亡途径,由先天性免疫传感器启动,并由 Caspases 和 RIPKs 通过 PANoptosomes 驱动。PANoptosomes 是由先天性免疫传感器针对病原体、PAMPs、DAMPs、细胞因子和驱动 PANoptosis 的稳态变化组装的多蛋白复合物。在这篇文章中,我们通过 Western 印迹、LDH 检测和显微镜读数,利用遗传和化学方法提供了分子定义不同细胞死亡途径(包括 PANoptosis)的方法。即使没有基因模型,这一程序也能在细胞群和单细胞水平上评估细胞死亡。所有实验室都能更方便地使用这种全面的工作流程,这将提高我们作为科学界加速发现的能力。使用这些方案将有助于鉴定驱动泛凋亡的新的先天性免疫传感器,并定义相关的分子机制和调控因子,从而建立临床转化的新靶点。© 2024 作者。当前协议》由 Wiley Periodicals LLC 出版。基本方案 1:利用活细胞成像诱导和量化细胞死亡 替代方案 1:利用 LDH 量化细胞死亡 替代方案 2:利用免疫荧光染色评估单细胞中的细胞死亡复合物 基本方案 2:利用免疫印迹(Western 印迹)分析细胞死亡机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。



