The ligamentum flavum (LF) degeneration is a critical factor in spinal stenosis, leading to nerve compression and pain. Even with new treatment options becoming available, it is vital to have a better understanding of LF degeneration to ensure the effectiveness of these treatments.
This study aimed to provide insight into LF degeneration by examining the connections between various aspects of LF degeneration, including histology, microstructure, chemical composition, and biomechanics.
We analyzed 30 LF samples from 27 patients with lumbar vertebrae, employing magnetic resonance imaging (MRI) to link lumbar disc degeneration grades with fibrosis levels in the tissue. X-ray diffraction (XRD) analysis assessed microstructural alterations in the LF matrix component due to degeneration progression. Instrumented nanoindentation combined with Raman spectroscopy explored the spatial microbiomechanical and biochemical characteristics of the LF's ventral and dorsal regions.
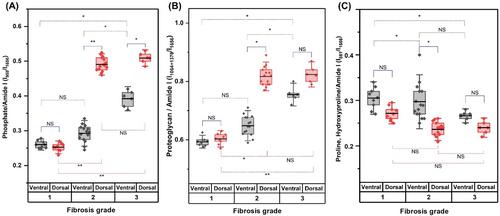
Our outcomes revealed a clear association between the severity of LF fibrosis grades and increasing LF thickness. XRD analysis showed a rise in crystalline components and hydroxyapatite molecules with progressing degeneration. Raman spectroscopy detected changes in the ratio of phosphate, proteoglycan, and proline/hydroxyproline over the amide I band, indicating alterations in the extracellular matrix composition. Biomechanical testing demonstrated that LF tissue becomes stiffer and less extensible with increasing fibrosis.
Notably, the micro-spatial assessment revealed the dorsal side of the LF experiencing more significant mechanical stress, alongside more pronounced biochemical and biomechanical changes compared to the ventral side. Degeneration of the LF involves complex processes that affect tissue histology, chemical composition, and biomechanics. It is crucial to fully understand these changes to develop new and effective treatments for spinal stenosis. These findings can improve diagnostic accuracy, identify potential biomarkers and treatment targets, guide personalized treatment strategies, advance tissue engineering approaches, help make informed clinical decisions, and educate patients about LF degeneration.