We examine levels of candidate blood-based biomarkers (CBBs) in patients with juvenile idiopathic arthritis (JIA) treated with tofacitinib.
Patients with JIA who participated in clinical trial NCT02592434 received tofacitinib from baseline to week 18. Serial serum samples were assayed for CBBs (S100A8/9, S100A12, interleukin-18 [IL-18], serum amyloid A, resistin, vascular endothelial growth factor, angiopoietin-1, angiopoietin-2, matrix metalloproteinase 8 [MMP8], MMP2, tissue inhibitor of metalloproteinases 1, leptin, chemokine [C-X-C motif] ligand 9, soluble IL-2 receptor, intercellular adhesion molecule 1, soluble tumor necrosis factor receptor, IL-6, IL-23, monocyte chemotactic protein 1, chemokine [C-C motif] ligand 18 [CCL18], and CCL20). Association of CBBs with JIA response to treatment from baseline to week 18 were assessed.
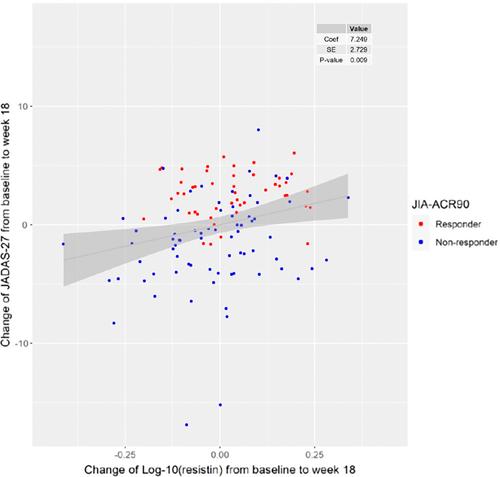
This study included 166 patients with polyarticular-course JIA. Paired serum samples from 143 patients were available at both baseline and week 18. Thirty-five percent (50 of 143) of patients had a JIA-American College of Rheumatology 90 (JIA-ACR90) level improvement, whereas 90, 121, and 137 (63%, 85%, and 96%) achieved JIA-ACR70, 50, and 30 improvement at week 18. Despite small numerical differences by JIA category, there were no baseline CBB values that independently predicted a decrease in Juvenile Arthritis Disease Activity Score (JADAS-27) or JIA-ACR90 response by week 18. Decrease in resistin level (baseline to week 18) was significantly associated with week 18 improvement in JADAS-27 and JIA-ACR90 response after adjusting for age, sex, JIA disease duration, and baseline resistin (r2 0.79, SE 0.070, P < 0.01, and odds ratio [95% confidence interval] 1.134 [1.018–1.264]). HLA-B27 positivity was significantly associated with not achieving a JIA-ACR90 response at week 18 (P = 0.0097).
Among the CBBs included, only resistin was significantly associated with treatment response, and no CBB was identified that forecasts JIA improvement after initiation of tofacitinib. The association of HLA-B27 positivity with lower response to tofacitinib in JIA is intriguing and merits further study.