Single-cell transcriptomic analysis reveals that the APP–CD74 axis promotes immunosuppression and progression of testicular tumors
Guo Chen, Wei Wang, Xin Wei, Yulin Chen, Liao Peng, Rui Qu, Yi Luo, Shengyin He, Yugao Liu, Jie Du, Ran Lu, Siying Li, Chuangwen Fan, Sujun Chen, Yi Dai, Luo Yang
下载PDF
{"title":"Single-cell transcriptomic analysis reveals that the APP–CD74 axis promotes immunosuppression and progression of testicular tumors","authors":"Guo Chen, Wei Wang, Xin Wei, Yulin Chen, Liao Peng, Rui Qu, Yi Luo, Shengyin He, Yugao Liu, Jie Du, Ran Lu, Siying Li, Chuangwen Fan, Sujun Chen, Yi Dai, Luo Yang","doi":"10.1002/path.6343","DOIUrl":null,"url":null,"abstract":"<p>Testicular tumors represent the most common malignancy among young men. Nevertheless, the pathogenesis and molecular underpinning of testicular tumors remain largely elusive. We aimed to delineate the intricate intra-tumoral heterogeneity and the network of intercellular communication within the tumor microenvironment. A total of 40,760 single-cell transcriptomes were analyzed, encompassing samples from six individuals with seminomas, two patients with mixed germ cell tumors, one patient with a Leydig cell tumor, and three healthy donors. Five distinct malignant subclusters were identified in the constructed landscape. Among them, malignant 1 and 3 subclusters were associated with a more immunosuppressive state and displayed worse disease-free survival. Further analysis identified that APP–CD74 interactions were significantly strengthened between malignant 1 and 3 subclusters and 14 types of immune subpopulations. In addition, we established an aberrant spermatogenesis trajectory and delineated the global gene alterations of somatic cells in seminoma testes. Sertoli cells were identified as the somatic cell type that differed the most from healthy donors to seminoma testes. Cellular communication between spermatogonial stem cells and Sertoli cells is disturbed in seminoma testes. Our study delineates the intra-tumoral heterogeneity and the tumor immune microenvironment in testicular tumors, offering novel insights for targeted therapy. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 3","pages":"250-269"},"PeriodicalIF":5.2000,"publicationDate":"2024-08-19","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6343","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://pathsocjournals.onlinelibrary.wiley.com/doi/10.1002/path.6343","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
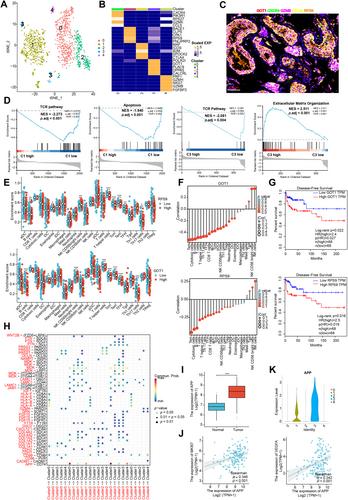
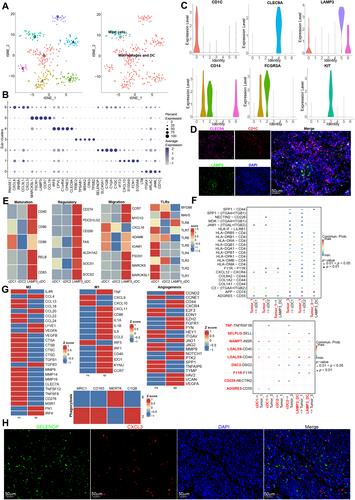
Testicular tumors represent the most common malignancy among young men. Nevertheless, the pathogenesis and molecular underpinning of testicular tumors remain largely elusive. We aimed to delineate the intricate intra-tumoral heterogeneity and the network of intercellular communication within the tumor microenvironment. A total of 40,760 single-cell transcriptomes were analyzed, encompassing samples from six individuals with seminomas, two patients with mixed germ cell tumors, one patient with a Leydig cell tumor, and three healthy donors. Five distinct malignant subclusters were identified in the constructed landscape. Among them, malignant 1 and 3 subclusters were associated with a more immunosuppressive state and displayed worse disease-free survival. Further analysis identified that APP–CD74 interactions were significantly strengthened between malignant 1 and 3 subclusters and 14 types of immune subpopulations. In addition, we established an aberrant spermatogenesis trajectory and delineated the global gene alterations of somatic cells in seminoma testes. Sertoli cells were identified as the somatic cell type that differed the most from healthy donors to seminoma testes. Cellular communication between spermatogonial stem cells and Sertoli cells is disturbed in seminoma testes. Our study delineates the intra-tumoral heterogeneity and the tumor immune microenvironment in testicular tumors, offering novel insights for targeted therapy. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
单细胞转录组分析显示,APP-CD74 轴促进了免疫抑制和睾丸肿瘤的进展。
睾丸肿瘤是年轻男性最常见的恶性肿瘤。然而,睾丸肿瘤的发病机制和分子基础在很大程度上仍然难以捉摸。我们的目标是描绘肿瘤微环境中错综复杂的瘤内异质性和细胞间通讯网络。我们共分析了 40,760 个单细胞转录组,包括来自 6 名精原细胞瘤患者、2 名混合生殖细胞瘤患者、1 名莱地格细胞瘤患者和 3 名健康供体的样本。在构建的图谱中发现了五个不同的恶性亚群。其中,恶性1和3亚群与免疫抑制状态相关,无病生存率较低。进一步分析发现,恶性1和3亚群与14种免疫亚群之间的APP-CD74相互作用明显增强。此外,我们还建立了精子发生异常的轨迹,并描述了精原细胞睾丸中体细胞的全局基因改变。我们发现,从健康捐献者到精原细胞瘤睾丸,塞尔托叶细胞是差异最大的体细胞类型。精原细胞干细胞和Sertoli细胞之间的细胞通讯在精原细胞瘤睾丸中受到干扰。我们的研究描述了睾丸肿瘤的瘤内异质性和肿瘤免疫微环境,为靶向治疗提供了新的见解。© 2024 作者姓名病理学杂志》由约翰威利父子有限公司代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。