Prostate cancer (PCa) is a highly heterogeneous disease, making tailored treatment approaches challenging. Magnetic resonance imaging (MRI), notably diffusion-weighted imaging (DWI) and the derived Apparent Diffusion Coefficient (ADC) maps, plays a crucial role in PCa characterization. In this context, radiomics is a very promising approach able to disclose insights from MRI data. However, the sensitivity of radiomic features to MRI settings, encompassing DWI protocols and multicenter variations, requires the development of robust and generalizable models.
To develop a comprehensive radiomics framework for noninvasive PCa characterization using ADC maps, focusing on identifying reliable imaging biomarkers against intra- and inter-institution variations.
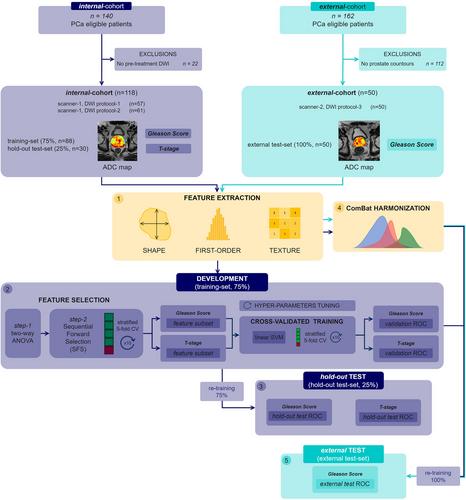
Two patient cohorts, including an internal cohort (118 PCa patients) used for both training (75%) and hold-out testing (25%), and an external cohort (50 PCa patients) for independent testing, were employed in the study. DWI images were acquired with three different DWI protocols on two different MRI scanners: two DWI protocols acquired on a 1.5-T scanner for the internal cohort, and one DWI protocol acquired on a 3-T scanner for the external cohort. One hundred and seven radiomics features (i.e., shape, first order, texture) were extracted from ADC maps of the whole prostate gland. To address variations in DWI protocols and multicenter variability, a dedicated pipeline, including two-way ANOVA, sequential-feature-selection (SFS), and ComBat features harmonization was implemented. Mann–Whitney U-tests (α = 0.05) were performed to find statistically significant features dividing patients with different tumor characteristics in terms of Gleason score (GS) and T-stage. Support-Vector-Machine models were then developed to predict GS and T-stage, and the performance was assessed through the area under the curve (AUC) of receiver-operating-characteristic curves.
Downstream of ANOVA, two subsets of 38 and 41 features stable against DWI protocol were identified for GS and T-stage, respectively. Among these, SFS revealed the most predictive features, yielding an AUC of 0.75 (GS) and 0.70 (T-stage) in the hold-out test. Employing ComBat harmonization improved the external-test performance of the GS model, raising AUC from 0.72 to 0.78.
By incorporating stable features with a harmonization procedure and validating the model on an external dataset, model robustness, and generalizability were assessed, highlighting the potential of ADC and radiomics for PCa characterization.