Single-cell transcriptomics identifies aberrant glomerular angiogenic signalling in the early stages of WT1 kidney disease
Jennifer C Chandler, Daniyal J Jafree, Saif Malik, Gideon Pomeranz, Mary Ball, Maria Kolatsi-Joannou, Alice Piapi, William J Mason, Andrew V Benest, David O Bates, Aleksandra Letunovska, Reem Al-Saadi, Marion Rabant, Olivia Boyer, Kathy Pritchard-Jones, Paul J Winyard, Andrew S Mason, Adrian S Woolf, Aoife M Waters, David A Long
下载PDF
{"title":"Single-cell transcriptomics identifies aberrant glomerular angiogenic signalling in the early stages of WT1 kidney disease","authors":"Jennifer C Chandler, Daniyal J Jafree, Saif Malik, Gideon Pomeranz, Mary Ball, Maria Kolatsi-Joannou, Alice Piapi, William J Mason, Andrew V Benest, David O Bates, Aleksandra Letunovska, Reem Al-Saadi, Marion Rabant, Olivia Boyer, Kathy Pritchard-Jones, Paul J Winyard, Andrew S Mason, Adrian S Woolf, Aoife M Waters, David A Long","doi":"10.1002/path.6339","DOIUrl":null,"url":null,"abstract":"<p><i>WT1</i> encodes a podocyte transcription factor whose variants can cause an untreatable glomerular disease in early childhood. Although WT1 regulates many podocyte genes, it is poorly understood which of them are initiators in disease and how they subsequently influence other cell-types in the glomerulus. We hypothesised that this could be resolved using single-cell RNA sequencing (scRNA-seq) and ligand-receptor analysis to profile glomerular cell–cell communication during the early stages of disease in mice harbouring an orthologous human mutation in WT1 (<i>Wt1</i><sup><i>R394W/+</i></sup>). Podocytes were the most dysregulated cell-type in the early stages of <i>Wt1</i><sup><i>R394W/+</i></sup> disease, with disrupted angiogenic signalling between podocytes and the endothelium, including the significant downregulation of transcripts for the vascular factors Vegfa and Nrp1. These signalling changes preceded glomerular endothelial cell loss in advancing disease, a feature also observed in biopsy samples from human WT1 glomerulopathies. Addition of conditioned medium from murine <i>Wt1</i><sup><i>R394W/+</i></sup> primary podocytes to wild-type glomerular endothelial cells resulted in impaired endothelial looping and reduced vascular complexity. Despite the loss of key angiogenic molecules in <i>Wt1</i><sup><i>R394W/+</i></sup> podocytes, the pro-vascular molecule adrenomedullin was upregulated in <i>Wt1</i><sup><i>R394W/+</i></sup> podocytes and plasma and its further administration was able to rescue the impaired looping observed when glomerular endothelium was exposed to <i>Wt1</i><sup><i>R394W/+</i></sup> podocyte medium. In comparative analyses, adrenomedullin upregulation was part of a common injury signature across multiple murine and human glomerular disease datasets, whilst other gene changes were unique to WT1 disease. Collectively, our study describes a novel role for altered angiogenic signalling in the initiation of WT1 glomerulopathy. We also identify adrenomedullin as a proangiogenic factor, which despite being upregulated in early injury, offers an insufficient protective response due to the wider milieu of dampened vascular signalling that results in endothelial cell loss in later disease. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 2","pages":"212-227"},"PeriodicalIF":5.2000,"publicationDate":"2024-08-23","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6339","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://pathsocjournals.onlinelibrary.wiley.com/doi/10.1002/path.6339","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
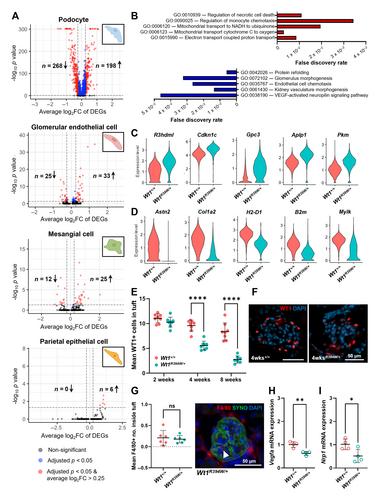
WT1 encodes a podocyte transcription factor whose variants can cause an untreatable glomerular disease in early childhood. Although WT1 regulates many podocyte genes, it is poorly understood which of them are initiators in disease and how they subsequently influence other cell-types in the glomerulus. We hypothesised that this could be resolved using single-cell RNA sequencing (scRNA-seq) and ligand-receptor analysis to profile glomerular cell–cell communication during the early stages of disease in mice harbouring an orthologous human mutation in WT1 (Wt1 R394W/+ Wt1 R394W/+ Wt1 R394W/+ Wt1 R394W/+ Wt1 R394W/+ Wt1 R394W/+ The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.