{"title":"Preparation of 2-Aminoimidazole-Activated Substrates for the Study of Nonenzymatic Genome Replication","authors":"James D. Robinson, Scott R. Sammons, Derek K. O'Flaherty","doi":"10.1002/cpz1.1119","DOIUrl":null,"url":null,"abstract":"<p>Nonenzymatic genome replication is thought to be an important process for primitive lifeforms, but this has yet to be demonstrated experimentally. Recent studies on the nonenzymatic primer extension mechanism mediated by nucleoside 5′-monophosphates (NMPs) activated with 2-aminoimidazole have revealed that imidazolium-bridged dinucleotide intermediates (N*N) account for the majority of the chemical copying process. As a result, an efficacious synthetic pathway for producing substrates activated with an imidazoyl moiety is desirable. This article provides a detailed protocol for the standard dehydrative redox reaction between NMPs and 2-aminoimidazole to produce nucleotide phosphoroimidazolides. In addition, we describe a similar synthetic pathway to produce N*N in high yields for homodimers. Finally, a simple reversed-phase cation exchange step is described to increase NMP solubility, which significantly increases yields for certain substrates. This approach allows for an efficient and cost-effective methodology to prepare high-quality substrates utilized in origins-of-life studies. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Synthesis of 2-aminoimidazolephosphoroimidazolide-activated cytidine</p><p><b>Basic Protocol 2</b>: Synthesis of 2-aminoimidazolium-bridged dicytidyl intermediate</p><p><b>Basic Protocol 3</b>: Cation exchange of guanosine 5′-monophosphate disodium salt</p><p><b>Alternate Protocol</b>: Synthesis of cytidine 5′-phosphoroimidazolide or 2-aminoimidazolium-bridged dicytidyl from cytidine 5′-monophosphate disodium salt</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 8","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2024-08-26","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1119","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.1119","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
Nonenzymatic genome replication is thought to be an important process for primitive lifeforms, but this has yet to be demonstrated experimentally. Recent studies on the nonenzymatic primer extension mechanism mediated by nucleoside 5′-monophosphates (NMPs) activated with 2-aminoimidazole have revealed that imidazolium-bridged dinucleotide intermediates (N*N) account for the majority of the chemical copying process. As a result, an efficacious synthetic pathway for producing substrates activated with an imidazoyl moiety is desirable. This article provides a detailed protocol for the standard dehydrative redox reaction between NMPs and 2-aminoimidazole to produce nucleotide phosphoroimidazolides. In addition, we describe a similar synthetic pathway to produce N*N in high yields for homodimers. Finally, a simple reversed-phase cation exchange step is described to increase NMP solubility, which significantly increases yields for certain substrates. This approach allows for an efficient and cost-effective methodology to prepare high-quality substrates utilized in origins-of-life studies. © 2024 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Synthesis of 2-aminoimidazolephosphoroimidazolide-activated cytidine
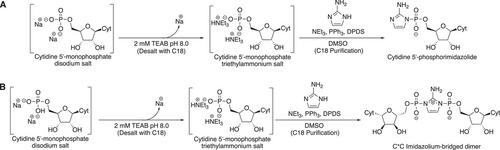
Basic Protocol 2: Synthesis of 2-aminoimidazolium-bridged dicytidyl intermediate
Basic Protocol 3: Cation exchange of guanosine 5′-monophosphate disodium salt
Alternate Protocol: Synthesis of cytidine 5′-phosphoroimidazolide or 2-aminoimidazolium-bridged dicytidyl from cytidine 5′-monophosphate disodium salt

制备用于研究非酶基因组复制的 2-Aminoimidazole-Activated Substrates。
非酶基因组复制被认为是原始生命形式的一个重要过程,但这一点尚未得到实验证明。最近对用 2-氨基咪唑激活的核苷 5'-单磷酸(NMP)介导的非酶引物延伸机制的研究表明,咪唑桥联二核苷酸中间体(N*N)占化学复制过程的大部分。因此,我们需要一种有效的合成途径来生产由咪唑酰基活化的底物。本文提供了 NMP 与 2-氨基咪唑进行标准脱水氧化还原反应生成核苷酸磷酰咪唑类化合物的详细方案。此外,我们还介绍了一种类似的合成途径,可以高产率生产 N*N 同二聚体。最后,我们介绍了一个简单的反相阳离子交换步骤,以增加 NMP 的溶解度,从而显著提高某些底物的产量。这种方法可以高效、经济地制备生命起源研究中使用的高质量底物。© 2024 作者。当前协议》由 Wiley Periodicals LLC 出版。基本方案 1:合成 2-氨基咪唑磷酰咪唑烷活化胞苷 基本方案 2:合成 2-氨基咪唑鎓桥接二胞苷中间体 基本方案 3:鸟苷-5'-单磷酸二钠盐的阳离子交换 替代方案:从胞苷 5'-单磷酸二钠盐合成胞苷 5'-磷酰基咪唑内酯或 2-氨基咪唑鎓桥接双胞苷。
本文章由计算机程序翻译,如有差异,请以英文原文为准。



