In recent years, minimally invasive biopsy techniques have been widely used to generate small tissue samples that require processing in clinical pathology. However, small paraffin-embedded tissues are prone to loss due to their small size. To prevent the loss of small tissues, researchers have employed nonbiological embedding materials for preembedding, but this approach can lead to cumbersome experimental procedures and increase the chances of tissue loss. This study aimed to develop a convenient decellularized embedding material derived from biological membrane tissues to effectively protect small tissues from loss during paraffin embedding. This study decellularized three types of fresh animal-derived membrane tissues and selected the small intestine as the most suitable decellularized raw material through attempts at softening, comparing physical properties, and using tissue as the starting material. Subsequently, small tissues from various tissue sources were embedded, followed by H&E staining, Masson staining, immunofluorescence staining, and immunohistochemical staining. The decellularized material derived from biomembrane tissues (DMBT) developed in this study can reduce the loss of small tissues without the need for preembedding, thereby shortening the embedding process. This provides a new pathological embedding tool for future laboratory and clinical research and work.
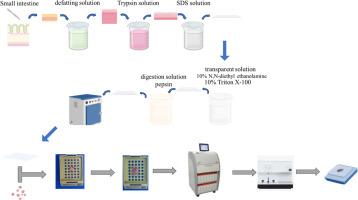
The fat layer of the pig's small intestine is scraped off, and chemical reagents are used to defat and decellularize it.
Chemical reagents are used to soften and make the pig's small intestine transparent, and the decellularized pig's small intestine is dried.
DMBT is used for embedding and staining the biological tissue.