This study explores molecular features associated with better prognosis in cholangiocarcinoma (CCA).
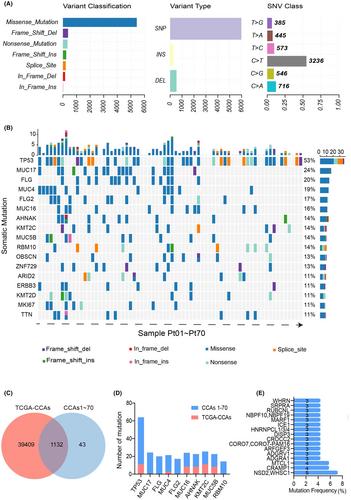
The transcriptomic and whole-exome sequencing data obtained from paired tissues of 70 were analyzed, grouping them based on progression-free survival (PFS), differentiation degree, and lymph node metastasis. Among the 70 patients, the TP53 gene mutation frequency was the highest (53%), while FLG gene mutation occurred exclusively in the long PFS group. In the comparison between long and short survival groups, the short PFS group exhibited higher monocyte infiltration levels (p = 0.0287) and upregulation of genes associated with cancer-related transcriptional misregulation, chemokine signaling, and cytokine-cytokine receptor interactions. Differences in immune cell infiltration and gene expression were significant across differentiation and lymph node metastasis groups. Particularly noteworthy was the marked increase in CD8 T cell and NK cell infiltration (p = 0.0291, 0.0459) in the lymph node metastasis group, significantly influences prognosis. Additionally, genes related to platinum resistance, Th17 cell differentiation, and Th1 and Th2 cell differentiation pathways were overexpressed in this group. In summary, higher monocyte infiltration levels in the short PFS group, along with elevated expression of genes associated with cancer-related pathways, suggest a poorer prognosis. The significant increase in CD8 T cell and NK cell infiltration reflects an enhanced anti-tumor immune response, underscoring the relevance of immune infiltration levels and gene expression in predicting outcomes for CCA patients.
In this study, we elucidated the pertinent molecular mechanisms and pathways that influence the prognosis of CCAs through comprehensive multi-omics analysis.