A Facile, Transfection-Free Approach to siRNA Delivery in In Vitro 3D Spheroid Models
Andrew S. Riching, Allyson Malloy, Emily M. Anderson, Jonathan Sheard, Piia Mikkonen, Anja van Brabant Smith, Zaklina Strezoska, Josien Levenga
{"title":"A Facile, Transfection-Free Approach to siRNA Delivery in In Vitro 3D Spheroid Models","authors":"Andrew S. Riching, Allyson Malloy, Emily M. Anderson, Jonathan Sheard, Piia Mikkonen, Anja van Brabant Smith, Zaklina Strezoska, Josien Levenga","doi":"10.1002/cpz1.1121","DOIUrl":null,"url":null,"abstract":"<p>Cell culture has long been essential for preclinical modeling of human development and disease. However, conventional two-dimensional (2D) cell culture fails to faithfully model the complexity found in vivo, and novel drug candidates that show promising results in 2D models often do not translate to the clinic. More recently, three-dimensional (3D) cell culture models have gained popularity owing to their greater physiological relevance to in vivo biology. In particular, 3D spheroid models are becoming widely used due to their ability to mimic solid tumors, both in architecture and gradation of nutrients distributed from the outer, proliferative layers into the inner, quiescent layers of cells. Similar to in vivo tumors, cell lines grown in 3D spheroid models tend to be more resistant to antitumor drug treatments than their 2D cultured counterparts, though distinct signaling pathways and gene targets conferring this resistance have yet to be fully explored. RNA interference (RNAi) is an effective tool to elucidate gene function and discover novel druggable targets in 2D models; however, only a few studies have successfully performed RNAi in complex 3D models to date. Here, we demonstrate efficient RNAi-mediated knockdown using “transfection-free” Dharmacon Accell siRNAs in three spheroid culture models, in the presence or absence of the extracellular matrix. This methodology has the potential to be scaled up for complex arrayed screening experiments, which may aid in the identification of novel druggable targets with greater clinical relevance than those identified in 2D experiments. © 2024 Dharmacon, Inc. Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Generation of 3D spheroids in matrix-free ULA plates</p><p><b>Alternate Protocol 1</b>: Generation of Matrigel matrix–embedded 3D spheroids</p><p><b>Alternate Protocol 2</b>: Generation of GrowDex hydrogel–embedded 3D spheroids</p><p><b>Basic Protocol 2</b>: Delivery of siRNA and collection of matrix-free 3D spheroids</p><p><b>Alternate Protocol 3</b>: Delivery of siRNA and collection of matrix-embedded spheroids</p><p><b>Basic Protocol 3</b>: RNA and protein extraction from spheroids for characterization of gene knockdown</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"4 9","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2024-09-03","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.1121","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.1121","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
Cell culture has long been essential for preclinical modeling of human development and disease. However, conventional two-dimensional (2D) cell culture fails to faithfully model the complexity found in vivo, and novel drug candidates that show promising results in 2D models often do not translate to the clinic. More recently, three-dimensional (3D) cell culture models have gained popularity owing to their greater physiological relevance to in vivo biology. In particular, 3D spheroid models are becoming widely used due to their ability to mimic solid tumors, both in architecture and gradation of nutrients distributed from the outer, proliferative layers into the inner, quiescent layers of cells. Similar to in vivo tumors, cell lines grown in 3D spheroid models tend to be more resistant to antitumor drug treatments than their 2D cultured counterparts, though distinct signaling pathways and gene targets conferring this resistance have yet to be fully explored. RNA interference (RNAi) is an effective tool to elucidate gene function and discover novel druggable targets in 2D models; however, only a few studies have successfully performed RNAi in complex 3D models to date. Here, we demonstrate efficient RNAi-mediated knockdown using “transfection-free” Dharmacon Accell siRNAs in three spheroid culture models, in the presence or absence of the extracellular matrix. This methodology has the potential to be scaled up for complex arrayed screening experiments, which may aid in the identification of novel druggable targets with greater clinical relevance than those identified in 2D experiments. © 2024 Dharmacon, Inc. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Generation of 3D spheroids in matrix-free ULA plates
Alternate Protocol 1: Generation of Matrigel matrix–embedded 3D spheroids
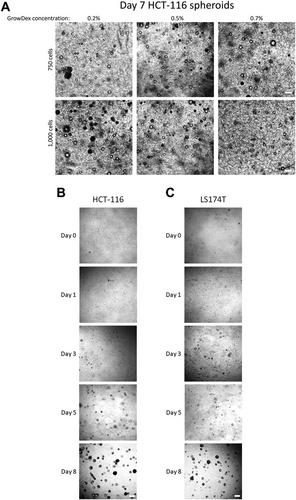
Alternate Protocol 2: Generation of GrowDex hydrogel–embedded 3D spheroids
Basic Protocol 2: Delivery of siRNA and collection of matrix-free 3D spheroids
Alternate Protocol 3: Delivery of siRNA and collection of matrix-embedded spheroids
Basic Protocol 3: RNA and protein extraction from spheroids for characterization of gene knockdown

在体外三维球状模型中传递 siRNA 的简便、免转染方法。
长期以来,细胞培养对于人类发育和疾病的临床前建模至关重要。然而,传统的二维(2D)细胞培养无法忠实地模拟体内的复杂性,在 2D 模型中显示出良好效果的候选新药往往无法应用于临床。最近,三维(3D)细胞培养模型因其与体内生物学更密切的生理相关性而越来越受欢迎。特别是三维球形模型,由于其能够模拟实体肿瘤的结构以及从外层增殖层到内层静止细胞的营养分布梯度,因此正得到广泛应用。与体内肿瘤类似,在三维球形模型中生长的细胞系往往比二维培养的细胞系对抗肿瘤药物治疗更有抵抗力,尽管导致这种抵抗力的独特信号通路和基因靶点还有待充分探索。RNA 干扰(RNAi)是在二维模型中阐明基因功能和发现新型药物靶点的有效工具;然而,迄今为止只有少数研究成功地在复杂的三维模型中实施了 RNAi。在这里,我们利用 "无转染 "的 Dharmacon Accell siRNAs,在有或没有细胞外基质的情况下,在三种球状培养模型中展示了高效的 RNAi 介导的基因敲除。这种方法有可能被放大用于复杂的阵列筛选实验,从而有助于发现比二维实验中发现的靶点更具临床意义的新型药物靶点。© 2024 Dharmacon, Inc.当前协议由 Wiley Periodicals LLC 出版。基本方案 1:在无基质 ULA 平板中生成三维球形体 替代方案 1:生成 Matrigel 基质包埋的三维球形体 替代方案 2:生成 GrowDex 水凝胶包埋的三维球形体 基本方案 2:递送 siRNA 并收集无基质的三维球形体 替代方案 3:递送 siRNA 并收集基质包埋的球形体 基本方案 3:从球形体中提取 RNA 和蛋白质以鉴定基因敲除。
本文章由计算机程序翻译,如有差异,请以英文原文为准。



