求助PDF
{"title":"Quercetin allylation and thiol–ene click photopolymerization to produce biobased polymer thermosets with robust thermomechanical properties","authors":"Víctor D Lechuga Islas, Ricardo Acosta Ortiz, Roberto Yañez Macías, Alan I Hernández Jiménez","doi":"10.1002/pi.6670","DOIUrl":null,"url":null,"abstract":"<p>The development of biobased and functionalized monomers along with eco-friendly photopolymerization processes represent promising methods for the development of renewable and more environmentally friendly thermosets. Thiol–ene ‘click’ photopolymerization is particularly advantageous in this regard; however, the materials derived from this method often exhibit low glass transition temperatures (<i>T</i><sub>g</sub>) and unsuitable thermomechanical properties for applications at room temperature. Herein, we report the synthesis of biobased allyl derivatives from quercetin, a renewable flavonoid compound widely available in fruits, vegetables and plants. We demonstrated the isolation of tetra- and penta-allylated quercetin (<b>Q1</b> and <b>Q2</b>, respectively) and their subsequent photoactivated thiol–ene polymerization. By introducing a biobased thiol curing agent (PTTMP) derived from glycerol and mercaptopropionic acid, we produced fully biobased crosslinked thermosets with high content of aromatic moieties provided by the framework of the monomers. Real-time infrared spectroscopy showed the effective thiol–ene photopolymerization of <b>Q1</b> and <b>Q2</b> and PTTMP with conversions of 60% and 75%, respectively, after 15 min of UV irradiation. Due to the modulated crosslinking degree from the allyl group functionalization in the monomers, the biobased crosslinked networks showed storage moduli from 420 to 739 MPa, thermal stability from 220 to 257 °C and <i>T</i><sub>g</sub> values ranging from 75 to 90 °C. This work outlines straightforward strategies for creating biobased thermosets that overcome the limited thermomechanical properties of thiol–ene networks and offer potential antimicrobial and antioxidant properties. © 2024 Society of Chemical Industry.</p>","PeriodicalId":20404,"journal":{"name":"Polymer International","volume":"73 10","pages":"864-873"},"PeriodicalIF":2.9000,"publicationDate":"2024-06-18","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Polymer International","FirstCategoryId":"92","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/pi.6670","RegionNum":4,"RegionCategory":"化学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"POLYMER SCIENCE","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
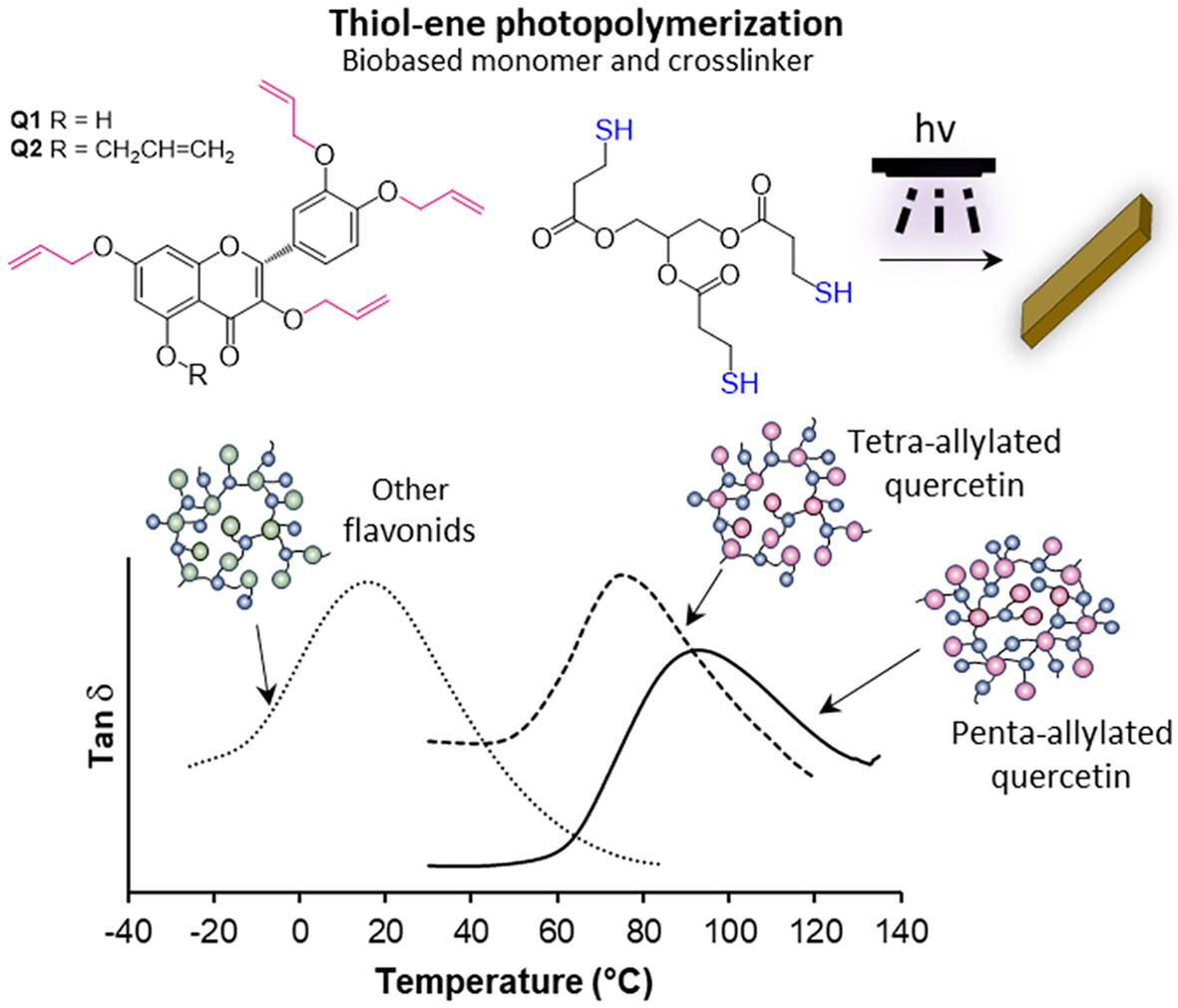
The development of biobased and functionalized monomers along with eco-friendly photopolymerization processes represent promising methods for the development of renewable and more environmentally friendly thermosets. Thiol–ene ‘click’ photopolymerization is particularly advantageous in this regard; however, the materials derived from this method often exhibit low glass transition temperatures (T g ) and unsuitable thermomechanical properties for applications at room temperature. Herein, we report the synthesis of biobased allyl derivatives from quercetin, a renewable flavonoid compound widely available in fruits, vegetables and plants. We demonstrated the isolation of tetra- and penta-allylated quercetin (Q1 and Q2 , respectively) and their subsequent photoactivated thiol–ene polymerization. By introducing a biobased thiol curing agent (PTTMP) derived from glycerol and mercaptopropionic acid, we produced fully biobased crosslinked thermosets with high content of aromatic moieties provided by the framework of the monomers. Real-time infrared spectroscopy showed the effective thiol–ene photopolymerization of Q1 and Q2 and PTTMP with conversions of 60% and 75%, respectively, after 15 min of UV irradiation. Due to the modulated crosslinking degree from the allyl group functionalization in the monomers, the biobased crosslinked networks showed storage moduli from 420 to 739 MPa, thermal stability from 220 to 257 °C and T g values ranging from 75 to 90 °C. This work outlines straightforward strategies for creating biobased thermosets that overcome the limited thermomechanical properties of thiol–ene networks and offer potential antimicrobial and antioxidant properties. © 2024 Society of Chemical Industry.
利用槲皮素烯丙基化和硫醇烯点击光聚合技术生产具有强热力学性能的生物基聚合物热固性塑料
生物基和功能化单体的开发以及环保型光聚合工艺是开发可再生和更环保热固性塑料的有效方法。在这方面,噻吩 "点击 "光聚合尤其具有优势;然而,这种方法制备的材料通常玻璃化温度(Tg)较低,热机械性能不适合在室温下应用。槲皮素是一种广泛存在于水果、蔬菜和植物中的可再生类黄酮化合物,在此,我们报告了从槲皮素合成生物基烯丙基衍生物的过程。我们展示了四烯丙基和五烯丙基槲皮素(分别为 Q1 和 Q2)的分离及其随后的光活化硫醇烯聚合。通过引入源自甘油和巯基丙酸的生物基硫醇固化剂(PTTMP),我们制备出了完全生物基的交联热固性塑料,其单体框架提供了高含量的芳香分子。实时红外光谱显示,在紫外线照射 15 分钟后,Q1 和 Q2 与 PTTMP 的硫醇-烯光聚合效果显著,转化率分别为 60% 和 75%。由于单体中的烯丙基官能化产生了可调节的交联度,生物基交联网络显示出 420 至 739 兆帕的存储模量、220 至 257 °C 的热稳定性和 75 至 90 °C 的 Tg 值。这项工作概述了创建生物基热固塑料的直接策略,这些热固塑料克服了硫醇烯网络有限的热机械性能,并具有潜在的抗菌和抗氧化性能。© 2024 化学工业协会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。