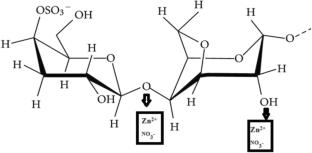
K-carrageenan and various concentrations of zinc nitrate hexahydrate have been combined to develop a zinc ion-conducting biopolymer electrolyte membrane using the solution casting method. To examine the amorphous nature of the samples, X-ray diffraction (XRD) study has been used. The maximum amorphous nature is observed for 1 g K-carrageenan with 1.1 M. wt % of zinc nitrate hexahydrate biopolymer membrane. Fourier transform infrared spectroscopy (FTIR) investigations have shown that the complexation occurs between the K-carrageenan with zinc nitrate hexahydrate. Surface morphology of Pure K-carrageenan, 1 g K-carrageenan with 1.1 M.wt % of zinc nitrate hexahydrate and 1 g K-carrageenan with 1.2 M.wt % of zinc nitrate hexahydrate have been studied by SEM analysis. Pure K-carrageenan membrane (SEM) has uniform surface with uniform small pores. 1 g K-carrageenan with 1.1 M.wt % of zinc nitrate hexahydrate membrane (SEM) shows rectangular rod-shaped nature along with pores of moderate diameter. 1 g K-carrageenan with 1.2 M.wt % of zinc nitrate hexahydrate membrane SEM shows rectangular rod-shaped nature along with pores of moderate diameter and aggregates. For the zinc-doped biopolymer membrane samples, the differential scanning calorimetry (DSC) is used to determine the glass transition temperature. Pure K-carrageenan has got Tg value at 37.04 °C. When the salt concentration is increased upto 0.9 M.wt % of the Tg value increases. When the salt concentration is further increased upto 1.1 M.wt % of the Tg value decreases. Highest zinc ion conducting membrane has got a Tg value of 75.95 °C. According to electrochemical impedance spectroscopy (EIS), the 1 g K-carrageenan with 1.1 M. wt % of zinc nitrate hexahydrate membrane has highest zinc ion conductivity of 2.9 × 10−3 S cm−1. According to the linear sweep voltammetry (LSV) investigation, the 1 g K-carrageenan with 1.1 M. wt % of zinc nitrate hexahydrate membrane has shown a wide electrochemical stability window of 2.75 V. The Evans polarization method determined that Zn2+ ion has a transference number of 0.42. The cyclic stability of highest conducting biopolymer membrane (Zn2+ ion) is studied by Cyclic Voltammetry. The electrolyte used in the construction of the primary zinc battery is 1 g K-carrageenan with 1.1 M. wt % of zinc nitrate hexahydrate having highest zinc ion conductivity. For this built-in battery, the OCV (Open Circuit Voltage) is found to be 1.43 V.