In MR-guided in-bore percutaneous needle interventions, typically 2D interactive real-time imaging is used for navigating the needle into the target. Misaligned 2D imaging planes can result in losing visibility of the needle in the 2D images, which impedes successful targeting. Necessary iterative manual slice adjustment can prolong interventional workflows. Therefore, rapid automatic alignment of the imaging planes with the needle would be preferable to improve such workflows.
To investigate rapid 3D localization of needles in MR-guided interventions via a convolutional neural network (CNN)-based localization algorithm using an undersampled white-marker contrast acquisition for the purpose of automatic imaging slice alignment.
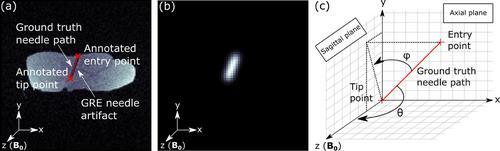
A radial 3D rf-spoiled gradient echo MR pulse sequence with white-marker encoding was implemented and a CNN-based localization algorithm was employed to extract position and orientation of an aspiration needle from the undersampled white-marker images. The CNN was trained using porcine tissue phantoms (257 needle trajectories, four-fold data augmentation, 90%/10% split into training and validation dataset). Achievable localization times and accuracy were evaluated retrospectively in an ex vivo study (109 needle trajectories) for a range of needle orientations between 78° and 90° relative to the B0 field. A proof-of-concept in vivo experiment was performed in two porcine animal models and feasibility of automatic imaging slice alignment was evaluated retrospectively.
Ex vivo needle localization was achieved with a median localization accuracy of 1.9 mm (distance needle tip to detected needle axis) and a median angular deviation of 2.6° for needle orientations between 86° and 90° to the B0 field from fully sampled WM images (resolution of (4 mm)3, 6434 acquired radial k-space spokes, acquisition time of 80.4 s) in a field-of-view of (256 mm)3. Localization accuracy decreased with increasing undersampling and needle trajectory increasingly aligned with B0. For needle orientations between 86° and 90° to the B0 field, a highly accelerated acquisition of only 32 k-space spokes (acquisition time of 0.4 s) yielded a median localization accuracy of 3.1 mm and a median angular deviation of 4.7°. For needle orientations between 78° and 82° to the B0 field, a median accuracy and angular deviation of 3.5 mm and 6.8° could still be achieved with 64 sampled spokes (acquisition time of 0.8 s). In vivo, a localization accuracy of 1.4 mm and angular deviation of 3.4° was achieved sampling 32 k-space spokes (acquisition time of 0.48 s) with the needle oriented at 87.7° to the B0 field. For a needle oriented at 77.6° to the B0 field, localization accuracy of 5.3 mm and angular deviation of 6.8° were still achieved sampling 128 k-space spokes (acquisition time of 1.92 s), allowing for retrospective slice alignment.
The investigated approach enables passive biopsy needle localization in 3D. Acceleration of the localization to real-time applicability is feasible for needle orientations approximately perpendicular to B0. The method can potentially facilitate MR-guided needle interventions by enabling automatic imaging slice alignment with the needle.