求助PDF
{"title":"Enhanced Electrocatalytic Activity for Nitrate Reduction to Ammonia by Tuning a Ruthenium Oxidation State of Ruthenium-Based Nanotubes","authors":"Xin Jiang, Pan-Yan Chen, Wan-Wan Wu, Jia-Yin Guo, Wei-Wei Li, Yu-Jie Mao, Tian Sheng, Xinsheng Zhao, Lu Wei","doi":"10.1021/acsanm.4c04066","DOIUrl":null,"url":null,"abstract":"Electrocatalytic nitrate reduction to ammonia (NRA) seriously suffers from slow kinetics and low selectivity due to its eight-electron transfer process and complex reaction intermediates. Herein, Ru-based nanotubes (NTs) were designed to enhance the electrocatalytic activity of NRA. Significantly, the metallic Ru NTs endowed remarkable ammonia (NH<sub>3</sub>) yield rate (<i></i><span style=\"color: inherit;\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\" display=\"inline\"><msub><mi>v</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub></math>' role=\"presentation\" style=\"position: relative;\" tabindex=\"0\"><nobr aria-hidden=\"true\"><span style=\"width: 2.162em; display: inline-block;\"><span style=\"display: inline-block; position: relative; width: 1.935em; height: 0px; font-size: 110%;\"><span style=\"position: absolute; clip: rect(1.537em, 1001.93em, 2.616em, -999.997em); top: -2.156em; left: 0em;\"><span><span><span style=\"display: inline-block; position: relative; width: 1.935em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1000.46em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Normal-italic;\">𝑣</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.804em; left: 0.514em;\"><span><span><span style=\"display: inline-block; position: relative; width: 1.31em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1001.03em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">NH</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.861em; left: 1.026em;\"><span style=\"font-size: 50%; font-family: STIXMathJax_Main;\">3</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 2.162em;\"></span></span></span><span style=\"display: inline-block; overflow: hidden; vertical-align: -0.372em; border-left: 0px solid; width: 0px; height: 0.941em;\"></span></span></nobr><span role=\"presentation\"><math display=\"inline\" xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub><mi>v</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub></math></span></span><script type=\"math/mml\"><math display=\"inline\"><msub><mi>v</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub></math></script>) of 40.6 mg h<sup>–1</sup> mg<sub>cat.</sub><sup>–1</sup> at −1.20 V vs SCE and the highest NH<sub>3</sub> Faradaic efficiency (<i></i><span style=\"color: inherit;\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\" display=\"inline\"><msub><mi>FE</mi><mrow><msub><mi mathvariant=\"normal\">NH</mi><mn>3</mn></msub></mrow></msub></math>' role=\"presentation\" style=\"position: relative;\" tabindex=\"0\"><nobr aria-hidden=\"true\"><span style=\"width: 2.844em; display: inline-block;\"><span style=\"display: inline-block; position: relative; width: 2.56em; height: 0px; font-size: 110%;\"><span style=\"position: absolute; clip: rect(1.31em, 1002.56em, 2.616em, -999.997em); top: -2.156em; left: 0em;\"><span><span><span style=\"display: inline-block; position: relative; width: 2.56em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.128em, 1001.14em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Main;\">FE</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.804em; left: 1.196em;\"><span><span><span style=\"display: inline-block; position: relative; width: 1.31em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1001.03em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">NH</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.861em; left: 1.026em;\"><span style=\"font-size: 50%; font-family: STIXMathJax_Main;\">3</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 2.162em;\"></span></span></span><span style=\"display: inline-block; overflow: hidden; vertical-align: -0.372em; border-left: 0px solid; width: 0px; height: 1.128em;\"></span></span></nobr><span role=\"presentation\"><math display=\"inline\" xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub><mi>FE</mi><mrow><msub><mi mathvariant=\"normal\">NH</mi><mn>3</mn></msub></mrow></msub></math></span></span><script type=\"math/mml\"><math display=\"inline\"><msub><mi>FE</mi><mrow><msub><mi mathvariant=\"normal\">NH</mi><mn>3</mn></msub></mrow></msub></math></script>) of 98.4% at −1.10 V vs SCE under ambient conditions, which are superior to those of RuO<sub>2</sub> NTs (<i></i><span style=\"color: inherit;\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\" display=\"inline\"><msub><mi>v</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub></math>' role=\"presentation\" style=\"position: relative;\" tabindex=\"0\"><nobr aria-hidden=\"true\"><span style=\"width: 2.162em; display: inline-block;\"><span style=\"display: inline-block; position: relative; width: 1.935em; height: 0px; font-size: 110%;\"><span style=\"position: absolute; clip: rect(1.537em, 1001.93em, 2.616em, -999.997em); top: -2.156em; left: 0em;\"><span><span><span style=\"display: inline-block; position: relative; width: 1.935em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1000.46em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Normal-italic;\">𝑣</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.804em; left: 0.514em;\"><span><span><span style=\"display: inline-block; position: relative; width: 1.31em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1001.03em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">NH</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.861em; left: 1.026em;\"><span style=\"font-size: 50%; font-family: STIXMathJax_Main;\">3</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 2.162em;\"></span></span></span><span style=\"display: inline-block; overflow: hidden; vertical-align: -0.372em; border-left: 0px solid; width: 0px; height: 0.941em;\"></span></span></nobr><span role=\"presentation\"><math display=\"inline\" xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub><mi>v</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub></math></span></span><script type=\"math/mml\"><math display=\"inline\"><msub><mi>v</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub></math></script>: 0.52 mg h<sup>–1</sup> mg<sub>cat.</sub><sup>–1</sup>, <i></i><span style=\"color: inherit;\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\" display=\"inline\"><msub><mi>FE</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub></math>' role=\"presentation\" style=\"position: relative;\" tabindex=\"0\"><nobr aria-hidden=\"true\"><span style=\"width: 2.844em; display: inline-block;\"><span style=\"display: inline-block; position: relative; width: 2.56em; height: 0px; font-size: 110%;\"><span style=\"position: absolute; clip: rect(1.31em, 1002.56em, 2.616em, -999.997em); top: -2.156em; left: 0em;\"><span><span><span style=\"display: inline-block; position: relative; width: 2.56em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.128em, 1001.14em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-family: STIXMathJax_Main;\">FE</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.804em; left: 1.196em;\"><span><span><span style=\"display: inline-block; position: relative; width: 1.31em; height: 0px;\"><span style=\"position: absolute; clip: rect(3.355em, 1001.03em, 4.151em, -999.997em); top: -3.974em; left: 0em;\"><span style=\"font-size: 70.7%; font-family: STIXMathJax_Main;\">NH</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span><span style=\"position: absolute; top: -3.861em; left: 1.026em;\"><span style=\"font-size: 50%; font-family: STIXMathJax_Main;\">3</span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 3.98em;\"></span></span></span></span></span><span style=\"display: inline-block; width: 0px; height: 2.162em;\"></span></span></span><span style=\"display: inline-block; overflow: hidden; vertical-align: -0.372em; border-left: 0px solid; width: 0px; height: 1.128em;\"></span></span></nobr><span role=\"presentation\"><math display=\"inline\" xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub><mi>FE</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub></math></span></span><script type=\"math/mml\"><math display=\"inline\"><msub><mi>FE</mi><mrow><msub><mi>NH</mi><mn>3</mn></msub></mrow></msub></math></script>: 18.2%). Both experimental and theoretical results have proved that the Ru metallic state is more beneficial to N–O bond breaking and hydrogenation than the oxidized state, improving the kinetics and selectivity of NRA.","PeriodicalId":6,"journal":{"name":"ACS Applied Nano Materials","volume":null,"pages":null},"PeriodicalIF":5.3000,"publicationDate":"2024-09-13","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"ACS Applied Nano Materials","FirstCategoryId":"88","ListUrlMain":"https://doi.org/10.1021/acsanm.4c04066","RegionNum":2,"RegionCategory":"材料科学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"MATERIALS SCIENCE, MULTIDISCIPLINARY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
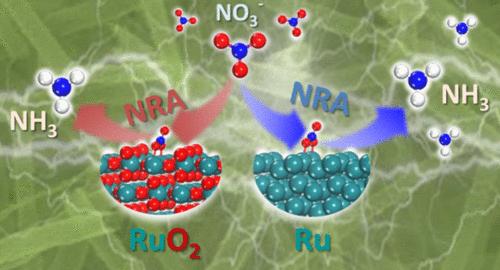
Electrocatalytic nitrate reduction to ammonia (NRA) seriously suffers from slow kinetics and low selectivity due to its eight-electron transfer process and complex reaction intermediates. Herein, Ru-based nanotubes (NTs) were designed to enhance the electrocatalytic activity of NRA. Significantly, the metallic Ru NTs endowed remarkable ammonia (NH
3 ) yield rate (
𝑣 NH 3 v NH 3 ) of 40.6 mg h
–1 mg
cat. –1 at −1.20 V vs SCE and the highest NH
3 Faradaic efficiency (
FE NH 3 FE NH 3 ) of 98.4% at −1.10 V vs SCE under ambient conditions, which are superior to those of RuO
2 NTs (
𝑣 NH 3 v NH 3 : 0.52 mg h
–1 mg
cat. –1 ,
FE NH 3 FE NH 3 : 18.2%). Both experimental and theoretical results have proved that the Ru metallic state is more beneficial to N–O bond breaking and hydrogenation than the oxidized state, improving the kinetics and selectivity of NRA.