Early-phases clinical trials (Phases 1 and 2) have evolved from a traditional assessment of toxicity to an adaptive approach based on patients' medical needs and access to effective new therapies. The global risks, benefits, and relevance of early-phases clinical trials participation for patients with hematological malignancies remain poorly evaluated.
All early-phases clinical trials participations for patients with hematological malignancies, from 2008 to 2023, in a tertiary academic center in Europe, were reviewed. Patient's demographics, tumor type categories, therapeutic responses, mortality, overall survival (OS), and investigational product (IP) were assessed.
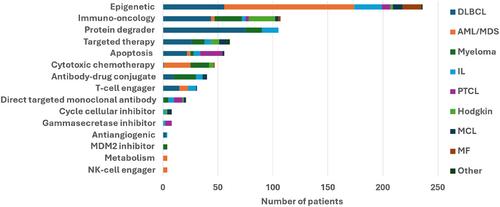
Over the period 2008–2023, 736 patients participating in 92 different early-phases clinical trials, were analyzed. The most common tumor categories were diffuse large B-cell lymphoma (n = 253; 34.4%), acute myeloid leukemia/myelodysplastic syndrome (n = 164; 22.3%) and multiple myeloma (n = 100; 13.6%). The median OS was 14.8 (95% CI: 12.4–17.9) months and response rate 31.9%, including complete responses in 13.5% of patients. By tumor categories, the highest and lowest median duration of OS were observed for patients with Hodgkin lymphoma (99.8; [95% CI: 47.0-not reached] months) and peripheral T-cell lymphoma (8.9 [95% CI: 5.3–12.0] months), respectively. The on-protocol and treatment-related mortality rates were 5.43% and 0.54%, respectively. Overall response rate was 29.1% including 13.5% of complete response. Overall, 202 (27.5%) patients received an IP later approved by the health authorities, and those patients had better OS (18.2 months vs. 12.1 months HR: 1.160 [95% CI; 0.6977–1.391], p = 0.0283).
In conclusion, patients with hematologic malignancies who have participated in early-phases clinical trials over the past 15 years have achieved variable therapeutic response rates, acceptable risk/benefit ratio and potentially significant therapeutic advantages. This study provides framework material for hematologists to further discuss clinical trial participation with their patients.