Surveillance approaches with high sensitivity and specificity for hepatocellular carcinoma (HCC) are still urgently needed. Previous studies have shown that methylation of GNB4 and Riplet can effectively diagnose HCC.
This study plan to analyze the performance of a blood test for detecting HCC using GNB4 and Riplet methylation.
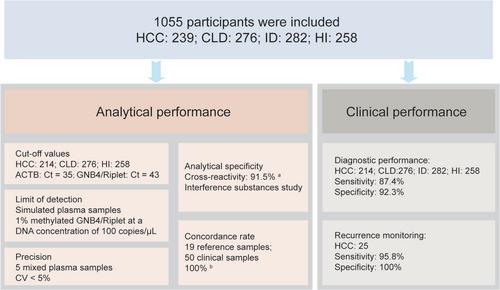
This study mainly investigated the analytical performance of the dual-target HCC blood test (DT-HBT), including cut-off value, limit of detection (LOD), precision, analytical specificity, and coincidence rate. In addition, the detection performance for HCC was validated in 1030 clinical plasma samples (214 HCC and 816 non-HCC). Plasma samples from 25 HCC patients after hepatectomy were collected to assess the feasibility of the kit for postoperative recurrence monitoring. All analytical performance of the DT-HBT met prespecified requirements. The LOD for GNB4, Riplet, and β-actin was 1% methylation/100 copies/μL with cut-offs of 43, 43, and 35, respectively. The DT-HBT showed excellent precision, within 5% CV. It had a specificity of 91.5% for detecting other cancers, and 100% for breast, lung, and bladder cancer. No cross-reactions were observed with 9 potential interfering substances. The DT-HBT achieved a 100% coincidence rate in detecting reference and clinical samples. The clinical performance study found that the kit showed a sensitivity of 81.7% for stage I HCC, and an overall sensitivity and specificity of 87.4% and 92.3%, respectively. The detection sensitivity for postoperative recurrent patients was 95.8%, with a specificity of 100%.
The analytical performance of the DT-HBT met prespecified criteria. It provided HCC patients with a reliable and high-performing new blood test for the HCC diagnosis and surveillance.
ClinicalTrials.gov identifier: NCT05685524