Genomic analysis defines distinct pancreatic and neuronal subtypes of lung carcinoid
Clara Domingo-Sabugo, Saffron AG Willis-Owen, Amit Mandal, Anca Nastase, Sarah Dwyer, Cecilia Brambilla, José Héctor Gálvez, Qinwei Zhuang, Sanjay Popat, Robert Eveleigh, Markus Munter, Eric Lim, Andrew G Nicholson, G Mark Lathrop, William OC Cookson, Miriam F Moffatt
下载PDF
{"title":"Genomic analysis defines distinct pancreatic and neuronal subtypes of lung carcinoid","authors":"Clara Domingo-Sabugo, Saffron AG Willis-Owen, Amit Mandal, Anca Nastase, Sarah Dwyer, Cecilia Brambilla, José Héctor Gálvez, Qinwei Zhuang, Sanjay Popat, Robert Eveleigh, Markus Munter, Eric Lim, Andrew G Nicholson, G Mark Lathrop, William OC Cookson, Miriam F Moffatt","doi":"10.1002/path.6352","DOIUrl":null,"url":null,"abstract":"<p>Lung carcinoids (L-CDs) are rare, poorly characterised neuroendocrine tumours (NETs). L-CDs are more common in women and are not the consequence of cigarette smoking. They are classified histologically as typical carcinoids (TCs) or atypical carcinoids (ACs). ACs confer a worse survival. Histological classification is imperfect, and there is increasing interest in molecular markers. We therefore investigated global transcriptomic and epigenomic profiles of 15 L-CDs resected with curative intent at Royal Brompton Hospital. We identified underlying mutations and structural abnormalities through whole-exome sequencing (WES) and single nucleotide polymorphism (SNP) genotyping. Transcriptomic clustering algorithms identified two distinct L-CD subtypes. These showed similarities either to pancreatic or neuroendocrine tumours at other sites and so were named respectively L-CD-PanC and L-CD-NeU. L-CD-PanC tumours featured upregulation of pancreatic and metabolic pathway genes matched by promoter hypomethylation of genes for beta cells and insulin secretion (<i>p</i> < 1 × 10<sup>−6</sup>). These tumours were centrally located and showed mutational signatures of activation-induced deaminase/apolipoprotein B editing complex activity, together with genome-wide DNA methylation loss enriched in repetitive elements (<i>p</i> = 2.2 × 10<sup>−16</sup>). By contrast, the L-CD-NeU group exhibited upregulation of neuronal markers (adjusted <i>p</i> < 0.01) and was characterised by focal spindle cell morphology (<i>p</i> = 0.04), peripheral location (<i>p</i> = 0.01), high mutational load (<i>p</i> = 2.17 × 10<sup>−4</sup>), recurrent copy number alterations, and enrichment for ACs. Mutations affected chromatin remodelling and SWI/SNF complex pathways. L-CD-NeU tumours carried a mutational signature attributable to aflatoxin and aristolochic acid (<i>p</i> = 0.05), suggesting a possible environmental exposure in their pathogenesis. Immunologically, myeloid and T-cell markers were enriched in L-CD-PanC and B-cell markers in L-CD-NeU tumours. The substantial epigenetic and non-coding differences between L-CD-PanC and L-CD-NeU open new possibilities for biomarker selection and targeted treatment of L-CD. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 3","pages":"332-343"},"PeriodicalIF":5.6000,"publicationDate":"2024-09-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6352","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6352","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
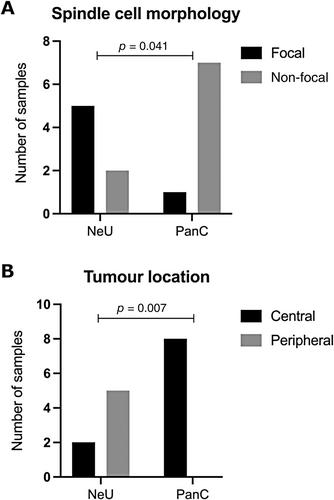
Lung carcinoids (L-CDs) are rare, poorly characterised neuroendocrine tumours (NETs). L-CDs are more common in women and are not the consequence of cigarette smoking. They are classified histologically as typical carcinoids (TCs) or atypical carcinoids (ACs). ACs confer a worse survival. Histological classification is imperfect, and there is increasing interest in molecular markers. We therefore investigated global transcriptomic and epigenomic profiles of 15 L-CDs resected with curative intent at Royal Brompton Hospital. We identified underlying mutations and structural abnormalities through whole-exome sequencing (WES) and single nucleotide polymorphism (SNP) genotyping. Transcriptomic clustering algorithms identified two distinct L-CD subtypes. These showed similarities either to pancreatic or neuroendocrine tumours at other sites and so were named respectively L-CD-PanC and L-CD-NeU. L-CD-PanC tumours featured upregulation of pancreatic and metabolic pathway genes matched by promoter hypomethylation of genes for beta cells and insulin secretion (p < 1 × 10−6 ). These tumours were centrally located and showed mutational signatures of activation-induced deaminase/apolipoprotein B editing complex activity, together with genome-wide DNA methylation loss enriched in repetitive elements (p = 2.2 × 10−16 ). By contrast, the L-CD-NeU group exhibited upregulation of neuronal markers (adjusted p < 0.01) and was characterised by focal spindle cell morphology (p = 0.04), peripheral location (p = 0.01), high mutational load (p = 2.17 × 10−4 ), recurrent copy number alterations, and enrichment for ACs. Mutations affected chromatin remodelling and SWI/SNF complex pathways. L-CD-NeU tumours carried a mutational signature attributable to aflatoxin and aristolochic acid (p = 0.05), suggesting a possible environmental exposure in their pathogenesis. Immunologically, myeloid and T-cell markers were enriched in L-CD-PanC and B-cell markers in L-CD-NeU tumours. The substantial epigenetic and non-coding differences between L-CD-PanC and L-CD-NeU open new possibilities for biomarker selection and targeted treatment of L-CD. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
基因组分析确定了肺类癌的不同胰腺亚型和神经元亚型。
肺类癌(L-CDs)是一种罕见、特征不明显的神经内分泌肿瘤(NETs)。类癌多见于女性,并非吸烟所致。它们在组织学上被分为典型类癌(TCs)和非典型类癌(ACs)。类癌的存活率较低。组织学分类并不完善,人们对分子标记物的兴趣与日俱增。因此,我们研究了皇家布朗普顿医院以治愈为目的切除的15例L-类癌的全球转录组和表观基因组图谱。我们通过全外显子组测序(WES)和单核苷酸多态性(SNP)基因分型确定了潜在的突变和结构异常。转录组学聚类算法确定了两种不同的 L-CD 亚型。这些亚型与其他部位的胰腺肿瘤或神经内分泌肿瘤有相似之处,因此被分别命名为 L-CD-PanC 和 L-CD-NeU。L-CD-PanC 肿瘤的特点是胰腺和代谢途径基因上调,与之相匹配的是β细胞和胰岛素分泌基因启动子的低甲基化(p-6)。这些肿瘤位于中心位置,显示出活化诱导脱氨酶/脂蛋白 B 编辑复合物活性的突变特征,以及富含重复元件的全基因组 DNA 甲基化缺失(p = 2.2 × 10-16)。相比之下,L-CD-NeU 组则表现出神经元标志物上调(调整后 p -4)、复发性拷贝数改变和 AC 的富集。突变影响染色质重塑和SWI/SNF复合体通路。L-CD-NeU肿瘤的突变特征可归因于黄曲霉毒素和马兜铃酸(p = 0.05),表明其发病机制可能与环境接触有关。在免疫学上,L-CD-PanC 肿瘤富含髓细胞和 T 细胞标记物,而 L-CD-NeU 肿瘤富含 B 细胞标记物。L-CD-PanC和L-CD-NeU在表观遗传学和非编码方面的巨大差异为L-CD的生物标记物选择和靶向治疗提供了新的可能性。© 2024 作者。病理学杂志》由 John Wiley & Sons Ltd 代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。