Spatial analysis of microRNA regulation at defined tumor hypoxia levels reveals biological traits of aggressive prostate cancer
Vilde E Skingen, Unn Beate Salberg, Tord Hompland, Christina S Fjeldbo, Hanna Helgeland, Kari-Anne M Frikstad, Harald B Ragnum, Ljiljana Vlatkovic, Knut Håkon Hole, Therese Seierstad, Heidi Lyng
下载PDF
{"title":"Spatial analysis of microRNA regulation at defined tumor hypoxia levels reveals biological traits of aggressive prostate cancer","authors":"Vilde E Skingen, Unn Beate Salberg, Tord Hompland, Christina S Fjeldbo, Hanna Helgeland, Kari-Anne M Frikstad, Harald B Ragnum, Ljiljana Vlatkovic, Knut Håkon Hole, Therese Seierstad, Heidi Lyng","doi":"10.1002/path.6344","DOIUrl":null,"url":null,"abstract":"<p>Mechanisms regulating the gene expression program at different hypoxia severity levels in patient tumors are not understood. We aimed to determine microRNA (miRNA) regulation of this program at defined hypoxia levels from moderate to severe in prostate cancer. Biopsies from 95 patients were used, where 83 patients received the hypoxia marker pimonidazole before prostatectomy. Forty hypoxia levels were extracted from pimonidazole-stained histological sections and correlated with miRNA and gene expression profiles determined by RNA sequencing and Illumina bead arrays. This identified miRNAs associated with moderate (<i>n</i> = 7) and severe (<i>n</i> = 28) hypoxia and predicted their target genes. The scores of miRNAs or target genes showed prognostic significance, as validated in an external cohort of 417 patients. The target genes showed enrichment of gene sets for cell proliferation and MYC activation at all hypoxia levels and PTEN inactivation at severe hypoxia. This was confirmed by RT-qPCR for <i>MYC</i> and <i>PTEN</i>, by Ki67 immunohistochemistry, and by gene set analysis in an external cohort. To assess whether miRNA regulation occurred within the predicted hypoxic regions, a method to quantify co-localization of multiple histopathology parameters at defined hypoxia levels was applied. A high Ki67 proliferation index co-localized significantly with hypoxia at all levels. The co-localization index was strongly associated with poor prognosis. Absence of PTEN staining co-localized significantly with severe hypoxia. The scores for miRNAs correlated with the co-localization index for Ki67 staining and hypoxia, consistent with miRNA regulation within the overlapping regions. This was confirmed by showing miR-210-3p expression within severe hypoxia by <i>in situ</i> hybridization. Cell line experiments (22Rv1, PC3) were conducted to determine whether miRNAs and target genes were regulated directly by hypoxia. Most of them were hypoxia-unresponsive, and probably regulated by other mechanisms such as MYC activation. In conclusion, in aggressive, hypoxic prostate tumors, cancer cells exhibit different proliferative gene expression programs that is regulated by miRNAs and depend on whether the cells reside in moderate or severe hypoxic regions. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 3","pages":"270-283"},"PeriodicalIF":5.2000,"publicationDate":"2024-09-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6344","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://pathsocjournals.onlinelibrary.wiley.com/doi/10.1002/path.6344","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
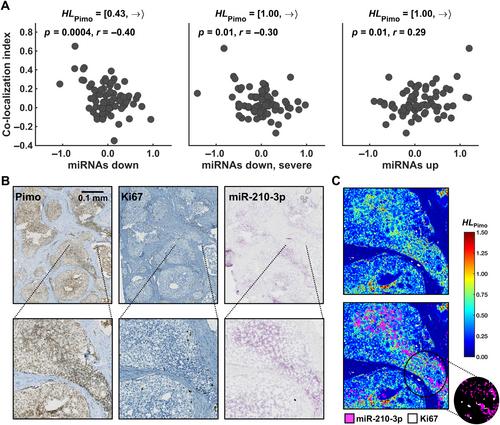
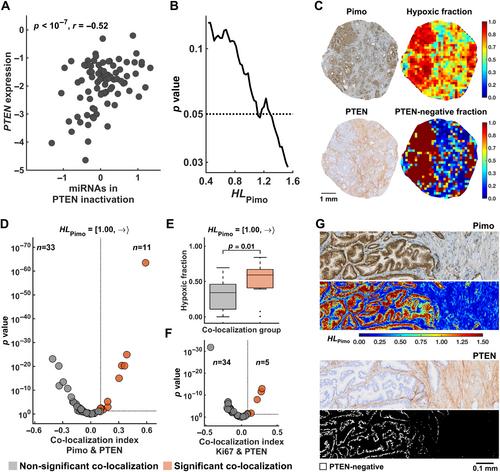
Mechanisms regulating the gene expression program at different hypoxia severity levels in patient tumors are not understood. We aimed to determine microRNA (miRNA) regulation of this program at defined hypoxia levels from moderate to severe in prostate cancer. Biopsies from 95 patients were used, where 83 patients received the hypoxia marker pimonidazole before prostatectomy. Forty hypoxia levels were extracted from pimonidazole-stained histological sections and correlated with miRNA and gene expression profiles determined by RNA sequencing and Illumina bead arrays. This identified miRNAs associated with moderate (n = 7) and severe (n = 28) hypoxia and predicted their target genes. The scores of miRNAs or target genes showed prognostic significance, as validated in an external cohort of 417 patients. The target genes showed enrichment of gene sets for cell proliferation and MYC activation at all hypoxia levels and PTEN inactivation at severe hypoxia. This was confirmed by RT-qPCR for MYC and PTEN , by Ki67 immunohistochemistry, and by gene set analysis in an external cohort. To assess whether miRNA regulation occurred within the predicted hypoxic regions, a method to quantify co-localization of multiple histopathology parameters at defined hypoxia levels was applied. A high Ki67 proliferation index co-localized significantly with hypoxia at all levels. The co-localization index was strongly associated with poor prognosis. Absence of PTEN staining co-localized significantly with severe hypoxia. The scores for miRNAs correlated with the co-localization index for Ki67 staining and hypoxia, consistent with miRNA regulation within the overlapping regions. This was confirmed by showing miR-210-3p expression within severe hypoxia by in situ hybridization. Cell line experiments (22Rv1, PC3) were conducted to determine whether miRNAs and target genes were regulated directly by hypoxia. Most of them were hypoxia-unresponsive, and probably regulated by other mechanisms such as MYC activation. In conclusion, in aggressive, hypoxic prostate tumors, cancer cells exhibit different proliferative gene expression programs that is regulated by miRNAs and depend on whether the cells reside in moderate or severe hypoxic regions. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
在确定的肿瘤缺氧水平下对微RNA调控的空间分析揭示了侵袭性前列腺癌的生物学特征。
目前还不清楚患者肿瘤中不同缺氧严重程度下基因表达程序的调控机制。我们的目的是确定前列腺癌患者在中度至重度缺氧水平下的微RNA(miRNA)对该程序的调控。我们使用了 95 例患者的活组织切片,其中 83 例患者在前列腺切除术前接受了缺氧标记物匹莫尼哒唑。从pimonidazole染色的组织学切片中提取了40个缺氧水平,并将其与通过RNA测序和Illumina珠阵列确定的miRNA和基因表达谱相关联。这样就确定了与中度(7 个)和重度(28 个)缺氧相关的 miRNA,并预测了它们的靶基因。经外部 417 例患者队列验证,miRNA 或靶基因的得分显示了预后意义。目标基因显示,在所有缺氧水平下,细胞增殖和MYC活化的基因集丰富,而在严重缺氧时,PTEN失活。MYC和PTEN的RT-qPCR、Ki67免疫组化以及外部队列的基因组分析均证实了这一点。为了评估 miRNA 的调控是否发生在预测的缺氧区域内,研究人员采用了一种方法来量化多个组织病理学参数在确定的缺氧水平下的共定位。高Ki67增殖指数在所有水平上都与缺氧显著共定位。共定位指数与预后不良密切相关。PTEN 染色缺失与严重缺氧显著共定位。miRNAs 的得分与 Ki67 染色和缺氧的共定位指数相关,这与重叠区域内的 miRNA 调控一致。通过原位杂交显示 miR-210-3p 在严重缺氧区域的表达,证实了这一点。为了确定缺氧是否直接调控 miRNA 和靶基因,进行了细胞系实验(22Rv1、PC3)。结果表明,大部分 miRNA 对缺氧无反应,可能受其他机制(如 MYC 激活)调控。总之,在侵袭性缺氧性前列腺肿瘤中,癌细胞表现出不同的增殖基因表达程序,这些程序受miRNAs调控,并取决于细胞是位于中度缺氧区域还是重度缺氧区域。© 2024 作者。病理学杂志》由 John Wiley & Sons Ltd 代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。