Alzheimer's disease is partially characterized by the progressive accumulation of aggregated tau-containing neurofibrillary tangles. Although the association between accumulated tau, neurodegeneration, and cognitive decline is critical for disease understanding and clinical trial design, we still lack robust tools to predict individualized trajectories of tau accumulation. Our objective was to assess whether brain imaging biomarkers of flortaucipir-positron emission tomography (PET), in combination with clinical and genomic measures, could predict future pathological tau accumulation.
We quantified the disease profile of participants (N = 276) using a comprehensive set of descriptors, including clinical/demographic (age, diagnosis, amyloid status, sex, race, ethnicity), genetic (apolipoprotein E [APOE]-ε4), and flortaucipir-PET imaging measures (regional flortaucipir standardized uptake value ratio [SUVr] and comprehensive radiomic texture features extracted from Automated Anatomical Labeling template regions). We trained an AdaBoost machine learning algorithm in a 2:1 split train-test configuration to derive a prognostic index that (i) stratifies individualized brain regions including global (AD-signature region) and lobar regions (frontal, occipital, parietal, temporal) into stable/slow- and fast-progressors based on future tau accumulation, and (ii) forecasts individualized regional annualized-rate-of-change in flortaucipir-PET SUVr. Further, we developed an adaptive model incorporating flortaucipir-PET measurements from the baseline and intermediate timepoints to predict annualized-rate-of-change.
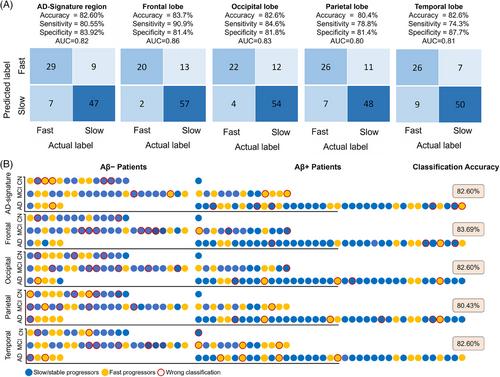
In binary classification for predicting stable/slow- versus fast-progressors, the area-under-the-receiver-operating-characteristic curve was 0.86 in the AD-signature region and 0.83, 0.82, 0.84, and 0.83 in frontal, occipital, parietal, and temporal regions, respectively. The trained models successfully predicted annualized-rate-of-change of flortaucipir-PET regional flortaucipir SUVr in AD-signature and lobar regions (Pearson-correlation [R]: AD-signature = 0.73; frontal = 0.73; occipital = 0.71; parietal = 0.70; temporal = 0.69). The models’ performance in predicting annualized-rate-of-change slightly increased when imaging features from intermediate timepoints were used in the adaptive setting (R: AD-signature = 0.79; frontal = 0.87; occipital = 0.83; parietal = 0.74; temporal = 0.82).
Taken together, our results propose a robust approach to predict future tau accumulation that may improve the ability to enroll, stratify, and gauge efficacy in clinical trial participants.